S-1: General form of registration statement for all companies including face-amount certificate companies
Published on September 5, 2023
As filed with the Securities and Exchange Commission on , 2023
Registration No.333 ‑
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D. C. 20549
FORM S‑1
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
| Know Labs, Inc. |
| (Exact name of registrant as specified in its charter) |
| Nevada |
| 3920 |
| 90‑0273142 |
| (State or other jurisdiction of incorporation or organization) |
| (Primary Standard Industrial Classification Code Number) |
| (IRS Employer Identification No.) |
500 Union Street, Suite 810
Seattle, Washington 98101
206‑903‑1351
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Ronald P. Erickson
Chief Executive Officer
500 Union Street, Suite 810
Seattle, Washington 98101
206‑903‑1351
(Names, address, including zip code and telephone number, including area code, of agent for service)
| Copies to: |
|
| Matthew S. O’Loughlin, Esq. Ben D. Orlanski, Esq. Louis Rambo, Esq. Proskauer Rose LLP 2029 Century Park East, Suite 2400 Los Angeles, CA 90067 (310) 284‑5653 | Cavas S. Pavri, Esq. ArentFox Schiff LLP 1717 K Street NW Washington, DC 20006 (202) 857-6000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post‑effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post‑effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b‑2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non‑accelerated filer | ☒ | Smaller reporting company | ☒ |
|
|
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission acting pursuant to such Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
SUBJECT TO COMPLETION, DATED , 2023
Preliminary Prospectus

$
Know Labs, Inc.
Shares of Common Stock
This is a firm commitment public offering. Pursuant to this prospectus, we are offering shares of our common stock , par value $0.001 per share. We currently estimate that the public offering price will be $ per share.
Our common stock is traded on the NYSE American under the symbol “KNW.” On , 2023, the last reported sale price of our common stock on the NYSE American was $ per share.
| ii |
You should read this prospectus, together with additional information described under the heading “Where You Can Find More Information,” carefully before you invest in any of our securities.
Investing in our securities involves a high degree of risk. See the section of this prospectus entitled “Risk Factors” beginning on page [7] for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
| Per Share |
|
| Total |
|
||
| Public offering price(1) |
| $ |
|
| $ |
|
||
| Underwriting discounts and commissions(2) |
| $ |
|
|
| $ |
|
|
| Proceeds to us (before expenses) |
| $ |
|
|
| $ |
|
|
|
| (1) | Based on an assumed public offering price of $ per share of common stock. The final public offering price per share of common stock will be determined by us, the underwriter and the purchasers in this offering and may be at a discount to the current market price of our common stock. |
|
|
|
|
|
| (2) | We have agreed to reimburse certain expenses of the underwriter which are not included in the table above and to issue the underwriter a warrant to purchase 7% of the shares of common stock issued in this offering. See “Underwriting” for a description of the compensation payable to the underwriter. |
We have granted the underwriter a 30-day option to purchase an aggregate of up to additional shares of our common stock at the public offering price per share of common stock less the underwriting discounts and commissions. The underwriter may exercise its option to acquire additional shares for the sole purpose of covering over-allotments. See “Underwriting.”
The underwriters are offering the shares on a firm commitment basis. The underwriters expect to deliver the shares to the purchasers on or about , 2023.
| Sole Book Running Manager |
| BOUSTEAD SECURITIES, LLC |
Prospectus dated , 2023
| iii |


| iv |

|
|
|
|
|
|
|
|
|
|
|
|
| April 21, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
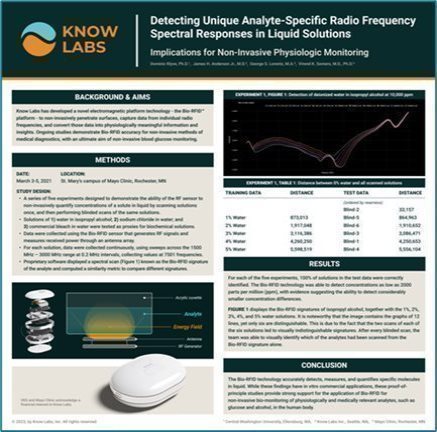
| • | Proof-of-principle conducted in collaboration with Mayo Clinic. |
|||
|
|
| • | Results presented at the American Physiological Society Summit, held on April 20-23 in Long Beach, California. |
|||
|
|
| • | Study demonstrated the accuracy of Bio-RFID sensor in quantifying three different analytes in vitro, proving a 100% accuracy rate in quantifying them. |
|||
|
|
| • | Full study is peer-reviewed and published at Sensors Journal |
|||
|
|
| • | Provides strong support for non-invasive monitoring of physiologically and medically relevant analytes in the body. |
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
* This study was performed in collaboration with Mayo Clinic, sponsored by the Company, and presented at the American Physiological Society (APS) Summit, which was held from April 20, 2023 to April 23, 2023 in Long Beach, California. This study was also published in Sensors Journal. Additional information can be found at “Bio-RFID: Validation and FDA Clearance” on page 36 of the publication.
| v |

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| May 5, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
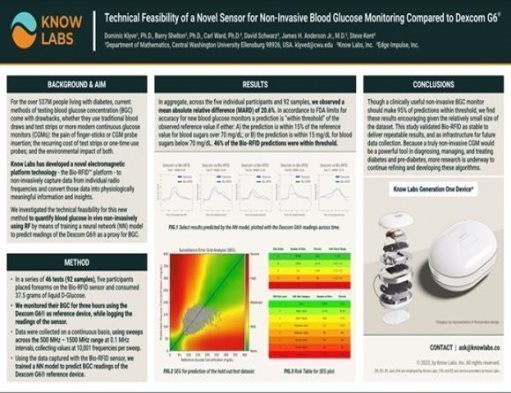
| • | Technical Feasibility of a Novel Sensor for Non-Invasive Blood Glucose Monitoring Compared to Dexcom G6® |
|||
|
| • | Presented at American Association of Clinical Endocrinology (AACE) 2023 Annual Meeting |
|||
|
| • | Demonstrates Bio-RFID sensor can deliver stable, repeatable results in predicting readings of blood glucose concentrations using the Dexcom G6® as a reference device |
|||
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* This study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle, and presented at at the American Association of Clinical Endocrinology (AACE) Annual Meeting on May 5, 2023 in Seattle, WA. Additional information can be found at “Bio-RFID: Validation and FDA Clearance” on page 36 of the publication.
| vi |

|
|
|
| July 26, 2023 |
|
||
|
|
|
|
|
|
|
|
|
| ||
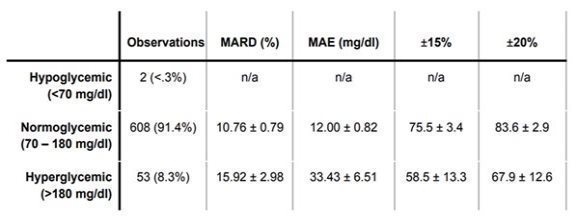
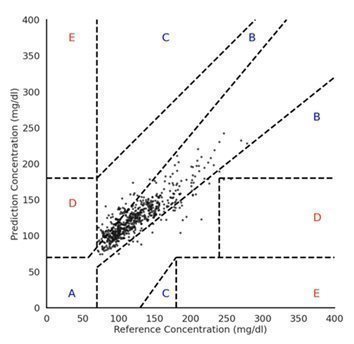
| • | Demonstrates a test in which the patented Bio-RFID sensor was able to predict reference values of a gold standard CGM (Dexcom G6) continuously and non-invasively with a MARD of 11.27% |
| ||
| • | One limitation is the requirement for a larger and more diverse participant population. All participants were healthy and did not have diabetes; indeed, 91.4% of the reference values were in the normoglycemic range |
| ||
* This study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle, and published at medRxiv, the pre-print server for health sciences on July 26, 2023. Additional information can be found at “Bio-RFID: Validation and FDA Clearance” on page 36 of the publication.
| vii |
|
|
| Page |
|
|
|
|
|
|
|
| 4 |
|
|
|
| 10 |
|
|
|
| 10 |
|
|
|
| 8 |
|
|
|
| 30 |
|
|
|
| 31 |
|
|
|
| 32 |
|
|
|
| 33 |
|
|
| Management’s Discussion And Analysis Of Financial Condition And Results Of Operations |
| 35 |
|
|
| 43 |
|
|
|
| 56 |
|
|
|
| 60 |
|
|
|
| 63 |
|
|
|
| 69 |
|
|
|
| 70 |
|
|
|
| 73 |
|
|
|
| 75 |
|
|
| Material United States Federal Income Tax Considerations For Holders Of Our Common Stock, |
| 81 |
|
|
| 85 |
|
|
|
| 88 |
|
|
|
| 88 |
|
|
|
| 89 |
|
|
|
| 90 |
|
| 1 |
| Table of Contents |
This prospectus constitutes a part of a registration statement on Form S‑1 (or, together with all amendments and exhibits thereto, the Registration Statement) filed by us with the Securities and Exchange Commission, or the SEC, under the Securities Act of 1933, as amended, or the Securities Act. As permitted by the rules and regulations of the SEC, this prospectus omits certain information contained in the Registration Statement, and reference is made to the Registration Statement and related exhibits for further information with respect to Know Labs, Inc. and the securities offered hereby. With regard to any statements contained herein concerning the provisions of any document filed as an exhibit to the Registration Statement or otherwise filed with the SEC, in each instance reference is made to the copy of such document so filed. Each such statement is qualified in its entirety by such reference.
You should rely only on the information contained in, or incorporated by reference into, this prospectus or in any related free‑writing prospectus. We and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectus prepared by us or on our behalf or to which we have referred you. We take no responsibility for and can provide no assurance as to the reliability of, any information that others may give you.
This prospectus is an offer to sell only the common stock offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. We are not making an offer to sell these shares of common stock in any jurisdiction where the offer or sale is not permitted or where the person making the offer or sale is not qualified to do so or to any person to whom it is not permitted to make such offer or sale. The information contained in this prospectus is accurate only as of the date of this prospectus and the information in the documents incorporated by reference herein is only accurate as of the respective dates of such documents, regardless of the time of delivery of this prospectus or of any sale of the securities registered hereby. Unless expressly stated otherwise, the information set forth in this prospectus supersedes any earlier dated information incorporated by reference herein. Our business, financial condition, operating results and prospects may have changed since that date.
Persons who come into possession of this prospectus and any applicable free writing prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus and any such free writing prospectus applicable to that jurisdiction. See “Underwriting” for additional information on these restrictions.
Until and including , 2023 (the 25th day after the date of this prospectus), all dealers effecting transactions in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
For investors outside of the United States: Neither we nor the underwriters have taken any action to permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
For purposes of this Registration Statement, “Company,” “we” or “our” refers to Know Labs, Inc. and its subsidiaries, unless otherwise required by the context.
| 2 |
| Table of Contents |
INDUSTRY AND MARKET DATA
This prospectus includes information with respect to market and industry conditions and market share from third-party sources or based upon estimates using such sources when available. We believe that such information and estimates are reasonable and reliable. We also believe the information extracted from publications of third-party sources has been accurately reproduced. However, we have not independently verified any of the data from third-party sources. Similarly, our internal research is based upon our understanding of industry conditions, and such information has not been verified by any independent sources.
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
We own or have rights to trademarks, service marks and trade names that we use in connection with the operation of our business, including our corporate name, logos and website names. Other trademarks, service marks and trade names appearing in this report are the property of their respective owners. Solely for convenience, some of the trademarks, service marks and trade names referred to in this report are listed without the ® and ™ symbols, but we will assert, to the fullest extent under applicable law, our rights to our trademarks, service marks and trade names. This report may include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included in this prospectus are the property of their respective owners.
| 3 |
| Table of Contents |
This summary highlights selected information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our securities. You should carefully read the entire prospectus, including the risks associated with an investment in our company discussed in the “Risk Factors” section of this prospectus, before making an investment decision. Some of the statements in this prospectus are forward‑looking statements. See the section titled “Cautionary Statement Regarding Forward‑Looking Statements.”
| OUR COMPANY
Overview
Know Labs is an emerging leader in non-invasive medical diagnostics. We are focused on the development and commercialization of our proprietary sensor technology utilizing radio and microwave spectroscopy. When paired with our machine learning platform, our technology is capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify, and measure the unique “signature” of said materials or analytes. We call this our “Bio-RFID™” sensor technology platform.
The first application of our Bio-RFID sensor technology is in a product to non-invasively monitor blood glucose levels. Our device will provide the user with real-time information on their blood glucose levels. We believe we would be the first non‑invasive glucose monitoring device available. We recently announced our Generation 1 working prototype device. This device embodies the Bio-RFID sensor which has been used in internal clinical testing. We will expand our testing, both internally and externally with the Generation 1 device and will refine the device itself over time into final form factors. These devices will require US Food and Drug Administration (FDA) clearance before entering the market because they will be considered to be medical devices.
Bio-RFID’s FDA clearance can open several recurring revenue opportunities for Know Labs, in addition to the revenue from device sales and/or licensing. We plan on exploring opportunities, which include but are not limited to, subscription as a service (SaaS), data as a service (DaaS), and Integration with other diagnostics solutions.
Following FDA clearance of our non-invasive blood glucose monitoring device, Know Labs also plans to expand Bio-RFID to other non-invasive medical diagnostic applications. As a platform technology, Bio-RFID can identify numerous other analytes in the human body that are important in medical diagnostics and human health and wellness.
While medical diagnostics applications are the focus of Know Labs with blood glucose monitoring paramount, the Company’s proprietary radio frequency and microwave spectroscopy platform have broad applicability outside of the medical diagnostic realm. Over time, as resources allow, the Company will explore those opportunities.
The Know Labs Technology
We have internally and under contract with third parties developed proprietary platform technology to uniquely identify and measure almost any organic and inorganic material or analyte. Our patented technology utilizes electromagnetic energy along a wide range of the electromagnetic spectrum from visible light and infrared to radio wave and microwave wavelengths to perform analytics that allow the user to accurately identify and measure materials and analytes.
Our technology provides a unique platform upon which a myriad of applications can be developed. As a platform technology, it is analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. Our radio frequency spectroscopy technology is an “enabling” technology that brings the science of electromagnetic energy to low-cost, real-world commercialization opportunities across multiple industries. The technology is foundational and, as such, the basis upon which we believe significant businesses can be built. While we are pursuing our core focus on commercializing our glucose monitor, we believe non-core clinical, non-clinical and medical research applications represent a multitude of opportunities for strategic collaboration, joint development, and licensing agreements with leading companies in their respective industries. |
| 4 |
| Table of Contents |
| Our Competitive Strengths
We believe our key competitive strengths include: |
||
|
|
|
|
|
| ● | Through first principles, Bio-RFID’s ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so non-invasively, and in real time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring. |
|
|
|
|
|
| ● | Our Bio-RFID technology platform can be integrated into a variety of wearable, mobile, or counter-top form factors, and we believe will provide interoperability with existing products from current market leaders. |
|
|
|
|
|
| ● | No needles nor invasive transmitters in your body, making Bio-RFID sensors convenient and pain-free. |
|
|
|
|
|
| ● | No expensive supplies, such as test strips and lancets, are required to operate Bio-RFID devices. |
|
|
|
|
|
| ● | A core focus on accessibility and affordability for the populations we will serve around the globe. |
|
|
|
|
|
| ● | The current prototype sensor collects approximately 1.5 million data points per hour, which allows Bio-RFID to potentially build a deep understanding of health and wellness that other sensors may not be able to. |
|
|
|
|
|
| ● | Know Labs is the world intellectual property leader in non-invasive blood glucose monitoring, according to ipCG Capital and PatSnap, with more than 150 patents issued and pending related to its core business. |
|
|
|
|
| Growth Strategy
The key elements of our strategy to grow our business include: |
||
|
|
||
|
| ● | Initially, entering the diabetes glucose monitoring market with our non-invasive glucose monitoring devices. |
|
|
|
|
|
| ● | We have selected the US as our first target market. However, more than 90% of the population with diabetes reside outside of the US. Following the regulatory clearance and commercial launch in the US, we plan on executing similar plan to other geographies. |
|
|
|
|
|
| ● | Following our entry into the glucose monitoring market, entering other clinical monitoring markets for continuous, non-invasive monitoring of other critical analytes, such as hormone, medication metabolites, endocrinology components, and biomolecular monitoring. |
|
|
|
|
|
| ● | Applying our Bio-RFID platform technology to lifestyle analysis, clinical trials, and chronic illnesses. We believe that potential use cases include real-time wearable medication monitoring and detection of, for example, ovulation and hormone deficiency. |
|
|
|
|
|
| ● | With an ever-growing body of non-invasively determined analytes available from individuals utilizing our Bio-RFID technology, we believe, over time, with longitudinal data that we will be able to engage in so-called “predictive health” and provide early warnings of the onset of disease. |
|
|
|
|
|
| ● | Significantly, every new application will function utilizing the same sensor. We expect that hardware changes will not be required to target new analytes so you will not need a new device, but an updated software algorithm will be required. |
|
|
|
|
|
| ● | Each new application provides potential new opportunities for monetization of the Bio-RFID platform technology. Each additional analyte we identify over time may require its own subsequent FDA approval if it is used in a medical device. |
|
|
|
|
|
| ● | While medical diagnostics applications are the focus of Know Labs, we believe our technology platform may have broad applicability outside of the medical diagnostic realm. As resources allow, the Company will explore those opportunities through strategic collaboration, joint development, and licensing agreements with leading companies in their respective industries. |
|
|
|
|
| Corporate Information
We were incorporated under the laws of the State of Nevada on October 8, 1998. Our executive office is located at 500 Union Street, Suite 810, Seattle, WA 98101. Our telephone number is (206) 903-1351 and our principal website address is located at www.knowlabs.co. The information on our website is not incorporated by reference in and is not deemed a part of this prospectus. |
||
| 5 |
| Table of Contents |
THE OFFERING
| Common stock offered by us:
|
| shares of common stock (or shares of common stock if the underwriters exercise the over-allotment option in full).
|
| Pre-funded warrants offered by us: |
| We are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, pre-funded warrants in lieu of shares of common stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant will be exercisable for one share of our common stock. The exercise price of each pre-funded warrant will equal $0.0001 per share. Each pre-funded warrant will be exercisable upon issuance and will not expire prior to exercise. We are offering up to shares of common stock and up to pre-funded warrants, in the aggregate not exceeding more than a total of shares of common stock and pre-funded warrants. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the pre-funded warrants.
|
| Common warrants offered by us: |
| We are issuing to purchasers of shares of our common stock and/or pre-funded warrants in this offering a common warrant to purchase up to one share of our common stock for each share and/or pre-funded warrant purchased in this offering for a combined purchase price of $ for one share and accompanying common warrant and $ for one pre-funded warrant and accompanying common warrant. Because a common warrant to purchase share(s) of our common stock is being sold together in this offering with each share of common stock and, in the alternative, each pre-funded warrant to purchase one share of common stock, the number of common warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold.
The common warrants will be exercisable on the date of closing at an exercise price of $ per share and will expire on the five-year anniversary of such closing. We are offering common warrants exercisable into an aggregate of up to shares of common stock in this offering. No fractional shares of common stock will be issued in connection with the exercise of a common warrant. In lieu of fractional shares, we will round down to the next whole share. See “Description of Securities — Common Warrants.” This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the common warrants.
|
| Offering price:
|
| We currently estimate that the public offering price will be $ per share.
|
| Common stock outstanding immediately before the offering:
|
| shares of common stock.
|
| Common stock outstanding immediately after the offering: |
| shares (or shares if the underwriters exercise the over-allotment option in full). |
| 6 |
| Table of Contents |
| Underwriting; Over-allotment option:
|
| We have granted the underwriter a 30-day option to purchase an aggregate of up to additional shares of our common stock from us at the public offering price per share of common stock less the underwriting discounts and commissions. The underwriter may exercise its option to acquire additional shares for the sole purpose of covering over-allotments. See “Underwriting.
Because our common stock is publicly traded, the underwriters may satisfy some or all of the overallotment of shares of our common stock, if any, by purchasing shares in the open market and will have no obligation to exercise the overallotment option with respect to our common stock. In that case, we will receive no proceeds from the exercise of the overallotment option.
|
| Use of proceeds:
|
| We estimate that the net proceeds from this offering will be approximately $ ($ if the underwriter’s option to purchase additional shares is exercised in full), based on an assumed public offering price per share of common stock of $ , the last reported sale price of our common stock on the NYSE American on , 2023 after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the proceeds from this offering for research and development, sales and marketing, general and administrative, capital investments and working capital. See “Use of Proceeds.”
|
| Risk factors:
|
| Investing in our securities involves a high degree of risk. As an investor, you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section beginning on page [7] as well as those risk factors in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, subsequent Quarterly Reports on Form 10-Q for the periods ended December 31, 2022, March 31, 2023, and June 30, 2023, and our other filings with the SEC, all of which are incorporated by reference herein before deciding to invest in our common stock.
|
| Lock-up:
|
| Our executive officers, directors and our security holder(s) of five percent (5%) or more have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any shares of our common stock for a lock-up period of six months following the closing of this offering, subject to certain exceptions. See “Underwriting” for more information.
|
| Trading symbol:
|
| Our common stock is traded on the NYSE American under the symbol “KNW.”
|
| No listing of pre-funded warrants or common warrants: |
| We do not intend to apply for listing of the pre-funded warrants or the common warrants on any national securities exchange or trading system. Without a trading market, the liquidity of the pre-funded warrants and the common warrants will be extremely limited. |
The number of shares of common stock outstanding immediately following this offering is based on 52,358,463 shares outstanding as of September 1, 2023 and excludes:
|
| ● | 14,506,158 shares of our common stock issuable upon the exercise of options which we granted to our officers, directors, and employees under the 2021 Plan (as defined below) at a weighted average exercise price of $1.546 per share (including unearned stock option grants totaling 3,869,825 shares related to performance milestones); |
|
|
|
|
|
| ● | 21,952,654 additional shares of our common stock that are reserved for issuance under the 2021 Plan; |
|
|
|
|
|
| ● | 8,108,356 shares of our common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock and approximately 2,920,000 common shares reserved to pay Series C and D preferred stock dividends, through June 30, 2023; |
|
|
|
|
|
| ● | 9,020,264 shares of our common stock issuable upon the conversion of convertible debentures; |
|
|
|
|
|
| ● | 18,856,313 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $1.15 per share; and |
|
|
|
|
|
| ● | up to ________ shares of our common stock issuable upon exercise of the representative’s warrant in connection with this offering. |
| 7 |
| Table of Contents |
CAUTIONARY STATEMENT REGARDING FORWARD‑LOOKING STATEMENTS
This prospectus contains forward‑looking statements that are based on our management’s beliefs and assumptions and on information currently available to us. All statements other than statements of historical facts are forward‑looking statements. The forward‑looking statements are contained principally in, but not limited to, the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward‑looking statements. Forward‑looking statements include, but are not limited to, statements about:
| ● | our goals and strategies; |
|
|
|
| ● | our future business development, financial condition and results of operations; |
|
|
|
| ● | expected product development outcomes, including obtaining regulatory clearance; |
|
|
|
| ● | expected changes in our revenue, costs or expenditures; |
|
|
|
| ● | growth of and competition trends in our industry; |
|
|
|
| ● | our expectations regarding demand for, and market acceptance of, our products; |
|
|
|
| ● | our expectations regarding our relationships with investors, institutional funding partners and other parties with whom we collaborate; |
|
|
|
| ● | our expectation regarding the use of proceeds from this offering; |
|
|
|
| ● | fluctuations in general economic and business conditions in the markets in which we operate; and |
|
|
|
| ● | relevant government policies and regulations relating to our industry. |
In some cases, you can identify forward‑looking statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward‑looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, subsequent Quarterly Reports on Form 10-Q for the periods ended December 31, 2022, March 31, 2023, and June 30, 2023, and our other filings with the SEC, all of which are incorporated by reference. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward‑looking statements. No forward‑looking statement is a guarantee of future performance.
The forward‑looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Although we will become a public company after this offering and have ongoing disclosure obligations under United States federal securities laws, we do not intend to update or otherwise revise the forward‑looking statements in this prospectus, whether as a result of new information, future events or otherwise.
| 8 |
| Table of Contents |
SUMMARY FINANCIAL INFORMATION
The following summary consolidated financial data as of and for the years ended September 30, 2022 and September 30, 2021 and nine months ended June 30, 2023 and June 30, 2022 are derived from our audited consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, and our unaudited consolidated financial statements included in our Quarterly Report on Form 10-Q for the period ended June 30, 2023, each of which is incorporated by reference herein. All financial statements included in this prospectus are prepared and presented in accordance with generally accepted accounting principles in the United States, or GAAP. You should read this data together with our consolidated financial statements and related notes included in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, which is incorporated by reference herein. Our historical results are not necessarily indicative of our future results and are not necessarily indicative of the results that may be expected for any interim periods, or any future year or period.
(dollars in thousands)
|
|
| Nine Months Ended June 30, |
|
|||||
|
|
| 2023 |
|
| 2022 |
|
||
|
|
| (Unaudited) |
|
| (Unaudited) |
|
||
| STATEMENT OF OPERATIONS DATA: |
|
|
|
|
|
|
||
| Net revenue |
| $ | - |
|
| $ | 4,360 |
|
| Research and development expenses |
|
| 6,186 |
|
|
| 3,407 |
|
| General and administrative expenses |
|
| 5,508 |
|
|
| 4,255 |
|
| Selling and transactional costs for digital assets |
|
| - |
|
|
| 3,437 |
|
| Total research and development and operating expenses |
|
| 11,694 |
|
|
| 11,099 |
|
| Operating loss |
|
| (11,694 | ) |
|
| (6,739 | ) |
| Total other (expense) income, net |
|
| (659 | ) |
|
| (7,762 | ) |
| Net loss before income taxes |
|
| (12,353 | ) |
|
| (14,501 | ) |
| Income tax expense |
|
| - |
|
|
| - |
|
| Net loss |
|
| (12,353 | ) |
|
| (14,501 | ) |
| Common stock dividends on Series D Preferred Stock |
|
| (1,627 | ) |
|
| - |
|
| Deemed dividends on Series C and D Preferred Stock |
|
| (3,338 | ) |
|
| - |
|
| Net loss available to common shareholders |
| $ | (17,318 | ) |
| $ | (14,501 | ) |
| Basic and diluted loss per share |
| $ | (0.36 | ) |
| $ | (0.37 | ) |
| Weighted average shares of common stock outstanding- basic and diluted |
|
| 48,604,274 |
|
|
| 39,032,860 |
|
(dollars in thousands)
|
|
| As of |
|
| As of September 30, |
|
||||||
|
|
| June 30, 2023 |
|
| 2022 |
|
| 2021 |
|
|||
| BALANCE SHEET DATA: |
| (Unaudited) |
|
|
|
|
|
|
|
|||
| Cash and cash equivalents |
| $ | 3,929 |
|
| $ | 12,594 |
|
| $ | 12,258 |
|
| Total current assets |
|
| 3,929 |
|
|
| 12,594 |
|
|
| 12,258 |
|
| Total assets |
|
| 4,436 |
|
|
| 13,758 |
|
|
| 12,889 |
|
| Total current liabilities |
|
| 3,721 |
|
|
| 3,809 |
|
|
| 11,037 |
|
| Total liabilities |
|
| 3,721 |
|
|
| 3,983 |
|
|
| 11,647 |
|
| Stockholders equity |
|
| 715 |
|
|
| 9,863 |
|
|
| 1,242 |
|
| Total liabilities and stockholders' equity |
| $ | 4,436 |
|
|
| 13,758 |
|
|
| 12,889 |
|
| 9 |
| Table of Contents |
An investment in our securities involves a high degree of risk. Before deciding whether to purchase our securities, including the shares of common stock offered by this prospectus, you should carefully consider the risks and uncertainties described under “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, subsequent Quarterly Reports on Form 10-Q for the periods ended December 31, 2022, March 31, 2023, and June 30, 2023, and our other filings with the SEC, all of which are incorporated by reference herein. If any of these risks actually occur, our business, financial condition and results of operations could be materially and adversely affected and we may not be able to achieve our goals, the value of our securities could decline and you could lose some or all of your investment. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations. If any of these risks occur, our business, results of operations or financial condition and prospects could be harmed. In that event, the market price of our common stock, and you could lose all or part of your investment. Some statements in this prospectus, including statements in the following risk factors, constitute forward-looking statements. Please refer to the section titled “Cautionary Statement Regarding Forward-Looking Statements.”
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. These risks are discussed more fully in the “Risk Factors” section immediately following this summary. These risks include, but are not limited to, the following:
Risks Related to Our Business and Industry
|
| ● | We might not be able to continue as a going concern. We believe that our cash on hand will be sufficient to fund our operations at least through December 31, 2023; |
|
|
|
|
|
| ● | We are still in the early stages of commercialization, refining our technology. Our success depends on our ability to conclude development and market devices that are recognized as accurate, safe, and cost-effective as other options currently available in the market and cleared by FDA. |
|
|
|
|
|
| ● | We are subject to extensive regulation by FDA, which could restrict the sales and marketing of our products and could cause us to incur significant costs; |
Risks Related to Ownership of Our Common Stock
|
| ● | The market price of our common stock may fluctuate, and you could lose all or part of your investment. |
|
|
|
|
|
| ● | We may not be able to maintain a listing of our common stock on the NYSE American. |
|
|
|
|
|
| ● | We do not expect to declare or pay dividends in the foreseeable future. |
|
|
|
|
|
| ● | Future issuances of our common stock or securities convertible into, or exercisable or exchangeable for, our common stock, or the expiration of lock-up agreements that restrict the issuance of new common stock or the trading of outstanding common stock, could cause the market price of our securities to decline and would result in the dilution of your holdings. |
|
|
|
|
|
| ● | Future issuances of debt securities, which would rank senior to our common stock upon our bankruptcy or liquidation, and future issuances of preferred stock, which could rank senior to our common stock for the purposes of dividends and liquidating distributions, may adversely affect the level of return you may be able to achieve from an investment in our common stock. |
| 10 |
| Table of Contents |
Risks Related to Our Business and Industry
We need the proceeds from this offering to continue as a going concern if our business is to succeed.
Because we have generated limited revenues and currently operate at a loss, we are completely dependent on the continued availability of financing in order to continue our business. There can be no assurance that financing sufficient to enable us to continue our operations will be available to us in the future.
As of June 30, 2023, we had cash and cash equivalents of $3,929,000 and net working capital of approximately $2,463,000 (exclusive of convertible notes payable of $2,255,000). We have experienced net losses since inception. As of June 30, 2023, we had an accumulated deficit of $118,715,000 and net losses in the amount of $12,353,000 and $20,071,000 and $25,360,000 during the nine months ended June 30, 2023 and the years ended September 30, 2022 and 2021, respectively. We incurred non-cash expenses of $3,454,000, $12,142,000, and $17,701,000 during the nine months ended June 30, 2023 and the years ended September 30, 2022 and 2021, respectively.
During the end of the quarter ended March 31, 2023, the Company made some adjustments to its staffing level, and the impact of those adjustments has significantly reduced our monthly burn rate. The Company will further adjust its cost structure if new debt or equity capital is not received. We believe that our cash on hand will be sufficient to fund our operations at least through December 31, 2023. As disclosed in the June 30, 2023 10-Q, as a result of not having at least twelve months of cash available and not having any firm commitment for debt or equity financing, substantial doubt about the Company’s ability to continue on a going concern exists.
We have financed our corporate operations and our technology development through the issuance of convertible debentures, the issuance of preferred stock, the sale of common stock and the exercise of warrants. During the remainder of 2023, we expect to raise additional funds through the issuance of preferred stock, convertible debentures or equity.
On September 20, 2022, we completed a public offering of our common stock pursuant to which we sold 4,140,000 shares of common stock, at a purchase price of $2.00 per share, for total gross proceeds of $8,280,000. After deducting underwriting commissions and other offering expenses, we received net proceeds of $7,425,000.
The proceeds of warrants currently outstanding, which are not exercised on a cashless basis, may generate potential proceeds of up to approximately $15,682,000. We cannot provide assurance that any of these warrants will be exercised.
As of June 30, 2023, we owed approximately $2,582,000 under various convertible promissory notes and other expenses, and if we do not satisfy these obligations, the lenders may have the right to demand payment in full or exercise other remedies.
We owe $2,255,000 under various convertible promissory notes as of June 30, 2023, including $1,071,000 to Clayton Struve who owns 100% of outstanding Series C and D Preferred stock, and $1,184,000 owed to entities controlled by Ronald P. Erickson, our Chairman and Chief Executive Officer. Mr. Erickson and/or entities with which he is affiliated also have accounts payable and accrued liabilities of $327,000 as of June 30, 2023 related accrued interest and expenses. We may need additional financing, to service and/or repay these debt obligations. If we raise additional capital through borrowing or other debt financing, we may incur substantial interest expense. If and when we raise more equity capital in the future, it will result in substantial dilution to our current stockholders.
| 11 |
| Table of Contents |
We have a history of operating losses and there can be no assurance that we can achieve or maintain profitability.
We have experienced net losses since inception. As of June 30, 2023, we had an accumulated deficit of $118,715,000 and net losses in the amount of $12,353,000, $20,071,000 and $25,360,000 during the nine months ended June 30, 2023 and the years ended September 30, 2022 and 2021, respectively. There can be no assurance that we will achieve or maintain profitability. If we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Failure to become and remain profitable would impair our ability to sustain operations and adversely affect the price of our common stock and our ability to raise capital. Our operating expenses may increase as we spend resources on growing our business, and if our revenue does not correspondingly increase, our operating results and financial condition will suffer. Our businesses have produced minimal revenues and may not produce significant revenues in the near term, or at all, which would harm our ability to continue our operations or obtain additional financing and require us to reduce or discontinue our operations. You must consider our business and prospects in light of the risks and difficulties we will encounter as business with an early-stage technology in a new and rapidly evolving industry. We may not be able to successfully address these risks and difficulties, which could significantly harm our business, operating results, financial condition and common stock price per share.
We may not be able to generate sufficient revenue from the commercialization of our technology and related products to achieve or sustain profitability.
We are in the early stages of commercializing our technology. Failure to develop and sell products based upon our technology could have a material adverse effect on our business, financial condition and results of operations. To date, we have not generated revenue from sales of our technology or products. We believe that our commercialization success is dependent upon our ability to significantly increase the number of customers that will use our products. In addition, demand for our products may not materialize, or increase as quickly as planned, and we may therefore be unable to increase our revenue levels as expected. We are currently not profitable. Even if we succeed in introducing our technology and related products to our target markets, we may not be able to generate sufficient revenue to achieve or sustain profitability.
We are subject to extensive regulation by the U.S. Food and Drug Administration, which could require us to take significant time and could cause us to incur significant costs.
Our the KnowU and UBand glucose monitoring products are subject to extensive regulation by FDA. These regulations relate to manufacturing, labeling, sale, promotion, distribution and shipping. Before a new medical device, or a new intended use of a legally marketed device, can be marketed in the United States, it must be cleared or approved by FDA through the applicable premarket review process (510(k), PMA, or de novo classification), unless an exemption applies.
The KnowU and UBand glucose monitoring products and substantially equivalent devices of this type that may later receive marketing authorization are similar to products referred to as integrated continuous glucose monitoring (CGM) systems. Integrated continuous glucose monitoring systems are generally classified by FDA as Class II devices and have established special controls outlining requirements for assuring CGM accuracy, reliability, and clinical relevance. FDA also has descriptions of the types of studies and data required to demonstrate acceptable CGM performance. Though it is our current belief that our initial product, the KnowU and UBand glucose monitoring products, are appropriate for a de novo classification request (i.e., a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device that is described in more detail below), we expect similar classification, special controls, and testing.
If we receive 510(k) clearance for our KnowU and UBand glucose monitoring products, we may be required to obtain new 510(k) clearances for significant post-market modifications. Each premarket submission and review process can be expensive and lengthy, and entail significant user fees, unless exempt. The classification and special controls for all other products using the Company’s proprietary radio frequency and microwave spectroscopy platform will be dependent on product type and explored as applicable.
| 12 |
| Table of Contents |
In addition, regulatory clearance or approval by FDA does not ensure registration, clearance, approval, or certification by regulatory authorities or notified bodies internationally. While the regulatory requirements for marketing in international markets may require that we obtain clearance, approval, or certification by an international specified regulatory body or notified body. Complying with foreign regulatory requirements, including obtaining registrations, clearances, approvals, or certifications, can be expensive and time consuming, and we may not receive regulatory clearances, approvals, or certifications in each country or region in which we plan to market our products or we may be unable to do so on a timely basis. In turn, this could limit our expected international growth and profitability, which could have a material adverse effect on our business, financial condition, and results of operations.
The clinical trial process is lengthy and expensive with uncertain outcomes. Results of earlier studies may not be predictive of future clinical trial results, or the safety or efficacy profile for such products.
Clinical trials are generally required to support an application for clearance of a new device type such as our KnowU and UBand glucose monitoring products. All clinical trials must be conducted in accordance with FDA’s Investigational Device Exemption (IDE) regulations, which govern investigational device labeling, prohibit promotion, and specify an array of Good Clinical Practice requirements, which include among other things, recordkeeping, reporting, and monitoring responsibilities of study sponsors and study investigators. Clinical trials must further comply with FDA’s regulations for institutional review board approval and for informed consent and other human subject protections. Required records and reports are subject to inspection by FDA.
Results of clinical testing may be unfavorable or, even if the intended safety and efficacy success criteria are achieved, may not be considered sufficient for FDA to grant approval or clearance of a product. In additional, the commencement or completion of any of our clinical trials may be delayed or halted for numerous reasons, including, but not limited to, the following:
|
| ● | we may be required to submit an investigational device exemption application, or IDE, to FDA, which must become effective prior to commencing certain human clinical trials of medical devices, and FDA may reject our IDE and notify us that we may not begin clinical trials; |
|
|
|
|
|
| ● | the cost of clinical trials may be greater than we anticipate; |
|
|
|
|
|
| ● | FDA or other regulatory authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold; |
|
|
|
|
|
| ● | patients do not enroll in clinical trials at the rate we expect; |
|
|
|
|
|
| ● | patients do not comply with trial protocols; |
|
|
|
|
|
| ● | patient follow-up is not at the rate we expect; |
|
|
|
|
|
| ● | patients experience adverse side effects; |
|
|
|
|
|
| ● | patients die during a clinical trial, even though their death may not be related to our products; |
|
|
|
|
|
| ● | we may not reach agreement on acceptable terms with prospective contract research organizations (CROs), and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
|
|
|
|
|
| ● | institutional review boards and third-party clinical investigators may delay or reject our trial protocol; |
|
|
|
|
|
| ● | third-party clinical investigators decline to participate in a trial or do not perform a trial on our anticipated schedule or consistent with the clinical trial protocol, good clinical practices, or other FDA requirements; |
| 13 |
| Table of Contents |
|
| ● | data collection, monitoring, and analysis is not performed in a timely or accurate manner or consistent with the clinical trial protocol or investigational or statistical plans; |
|
|
|
|
|
| ● | regulatory inspections of our clinical trials or manufacturing facilities, which may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials; |
|
|
|
|
|
| ● | changes in governmental regulations or administrative actions applicable to our trial protocols, including, for example, recent legislation passed by Congress requiring clinical trial sponsors to increase engagement with FDA on matters related to appropriate representation of racial and ethnic minorities in clinical trial data for pivotal studies; |
|
|
|
|
|
| ● | the interim or final results of the clinical trial are inconclusive or unfavorable as to safety or effectiveness; and |
|
|
|
|
|
| ● | FDA concludes that the results from our trial and/or trial design are inadequate to demonstrate safety and effectiveness of the product. |
Additionally, the ability of FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel, the availability of industry-paid user fees, and statutory, regulatory, and policy changes. Average review times for product approvals at FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at FDA and other agencies, including those resulting from global concerns (e.g., the ongoing COVID-19 global pandemic), may also slow the time necessary for new products to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, if a prolonged government shutdown and/or government employee furloughs were to occur, or if FDA’s response to a global issue diverts FDA resources and attention to other regulatory efforts, then the ability of FDA to timely review and process our regulatory submissions could be significantly impacted, which could have a material adverse effect on our business, financial condition, and results of operations. Further, in our operations as a public company, future government shutdowns, furloughs, or public health emergencies could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Any of these occurrences may significantly harm our business, financial condition, and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Moreover, even if our products are cleared in the U.S., commercialization of our products in foreign countries would require clearance or approval by regulatory authorities in those countries. Clearance or approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials.
The safety and efficacy of our products is not yet supported by long-term clinical data, which could limit sales, and our products might therefore prove to be less safe or effective than initially thought.
Given the regulatory environment in which we operate, we lack the breadth of published long-term clinical data supporting the safety and efficacy of The KnowU and UBand glucose monitoring products and the benefits it offers that might have been generated in connection with other marketing authorization pathways. For these reasons, clinicians may be slow to adopt our products, we may not have comparative data that our competitors have or are generating, and we may be subject to greater regulatory and product liability risks. Further, future patient studies or clinical experience may indicate that treatment with our product does not improve patient outcomes. Such results would slow the adoption of our product by physicians, would significantly reduce our ability to achieve expected sales, and could prevent us from achieving and maintaining profitability.
| 14 |
| Table of Contents |
In addition, because the KnowU and UBand glucose monitoring products have never been marketed, we have limited complaints or patient success rate data with respect to using these products. If future patient studies or clinical testing do not support our belief that our products offer a more advantageous blood glucose monitoring, then market acceptance of our products could fail to increase or could decrease, and our business could be harmed. Moreover, if future results and experience indicate that our product has potentially recurring malfunctions or causes unexpected or serious complications or other unforeseen negative effects, then we could be subject to mandatory or voluntary product recalls, suspension or withdrawal of FDA clearance, as well as significant legal liability or harm to our business reputation and financial results.
If we choose to, or are required to, conduct additional clinical studies and the outcome of such studies are not positive, then this could reduce the rate of coverage and reimbursement for the KnowU and UBand glucose monitoring products. This may slow the market adoption of our product by physicians, significantly reduce our ability to achieve expected revenues and prevent us from becoming profitable.
We believe that publications of scientific and medical results in peer-reviewed journals and presentations at leading conferences are critical to the broad adoption of our products. Publication in leading medical journals is subject to a peer-review process, and peer reviewers may not consider the results of studies involving our products sufficiently novel or worthy of publication. The failure to be listed in physician guidelines or to be published in peer-reviewed journals could limit the adoption of our products. Unless specifically stated to be “peer-reviewed,” the studies referred to in this prospectus are not peer reviewed.
We are subject to extensive regulation which could restrict the sales and marketing of our products and could cause us to incur significant costs.
Medical devices may be marketed only for the indications for which they are approved or cleared. Further, clearances can be revoked if safety or effectiveness problems develop once the device is on the market.
The current regulatory requirements to which we are subject may change in the future in a way that adversely affects us. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by FDA, which may include any of the following sanctions:
|
| ● | modification to our training and promotional materials; |
|
| ● | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
| ● | customer notification, or orders for repair, replacement or refunds; |
|
| ● | voluntary or mandatory recall or seizure of our current or future products; |
|
| ● | administrative detention by FDA of medical devices believed to be adulterated or misbranded; |
|
| ● | imposing operating restrictions, suspension or shutdown of production; |
|
| ● | refusing our requests for clearance, PMA or de novo classification of any new products, new intended uses or modifications to our products; |
|
| ● | FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; |
|
| ● | withdraws or suspension of 510(k) clearance that has already been granted, resulting in prohibitions on sales of our products; and |
|
| ● | criminal prosecution. |
The occurrence of any of these events would have a material adverse effect on our business, financial condition and results of operations and could result in stockholders losing their entire investment.
| 15 |
| Table of Contents |
Additionally, any relationships we may have with healthcare professionals, clinical investigators, and payors in connection with our current and future business activities may be subject to federal and state healthcare fraud and abuse laws, false claims laws, transparency laws, and health information privacy and security laws, which could expose us to, among other things, criminal sanctions, civil penalties, contractual damages, exclusion from governmental healthcare programs, reputational harm, administrative burdens, and diminished profits and future earnings.
Healthcare providers and payors play a primary role in the recommendation and/or prescription of any product candidates for which we obtain future marketing approval. Our current and future arrangements with healthcare professionals, clinical investigators, payors, and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell, and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
|
| ● | the federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act; |
|
|
|
|
|
| ● | the federal false claims and civil monetary penalties laws, including the civil False Claims Act, which can be enforced by private citizens through civil whistleblower or qui tam actions, prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government. The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, prohibits, among other things, executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
|
|
|
|
|
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information; |
|
|
|
|
|
| ● | the federal Physician Payments Sunshine Act requires applicable manufacturers of covered drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to annually report to Centers for Medicare & Medicaid Services (CMS) starting in 2022 information regarding payments and other transfers of value to physicians, certain other healthcare providers, and teaching hospitals, as well as information regarding ownership and investment interests held by physicians and their immediate family members. The information reported will be publicly available on a searchable website, with disclosure required annually; and |
|
|
|
|
|
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. |
State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. For instance, the collection and use of health data in the European Union is governed by the General Data Protection Regulation, or the GDPR, which extends the geographical scope of European Union data protection law to non-European Union entities under certain conditions, tightens existing European Union data protection principles, creates new obligations for companies and new rights for individuals. Failure to comply with the GDPR may result in substantial fines and other administrative penalties. In addition, on June 28, 2018, the State of California enacted the California Consumer Privacy Act, or CCPA, which took effect on January 1, 2020. The CCPA creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability, and similar laws have been proposed at the federal level and in other states.
| 16 |
| Table of Contents |
Efforts to ensure that our current and future business arrangements with third parties will comply with applicable healthcare laws and regulations will involve on-going substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, then we may be subject to significant penalties, including civil, criminal, and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, temporary or permanent debarment, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations. Defending against any such actions can be costly, time-consuming, and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. Further, if any of the physicians or other healthcare providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, then they may be subject to criminal, civil, or administrative sanctions, including exclusions from government funded healthcare programs.
A variety of risks associated with marketing our product candidates internationally could materially adversely affect our business.
We may seek regulatory approval of our product candidates outside of the U.S., and, accordingly, we expect that we will be subject to additional risks related to operating in foreign countries if we obtain the necessary approvals, including:
|
| · | differing regulatory requirements and reimbursement regimes in foreign countries; |
|
|
|
|
|
| · | unexpected changes in tariffs, trade barriers, price and exchange controls, and other regulatory requirements; |
|
|
|
|
|
| · | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
|
|
|
|
|
| · | compliance with tax, employment, immigration, and labor laws for employees living or traveling abroad; |
|
|
|
|
|
| · | foreign taxes, including withholding of payroll taxes; |
|
|
|
|
|
| · | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
|
|
|
|
|
| · | difficulties staffing and managing foreign operations; |
|
|
|
|
|
| · | workforce uncertainty in countries where labor unrest is more common than in the U.S.; |
|
|
|
|
|
| · | potential liability under the Foreign Corrupt Practices Act (FCPA) or comparable foreign regulations; |
|
|
|
|
|
| · | challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the U.S.; |
|
|
|
|
|
| · | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
|
|
|
|
|
| · | business interruptions resulting from geo-political actions, including war and terrorism. |
| 17 |
| Table of Contents |
These and other risks associated with our international operations may materially adversely affect our ability to attain or maintain profitable operations.
We may face difficulties with respect to coverage and reimbursement from by various payors.
Sales of any medical device depend often, in part, on the extent to which the product will be covered and reimbursed by government payors (e.g., federal and state healthcare programs), third-party payors (e.g., commercial insurance and managed healthcare organizations), and other payors (e.g., foreign government healthcare programs). In the United States, various glucose monitoring products are covered for individuals with both Type 1 and Type 2 diabetes by Medicare and Medicaid in the majority of states and by commercial insurers, subject to satisfaction of certain eligibility and coverage criteria.
But significant uncertainty exists as to the coverage and reimbursement status of any newly approved product. For example, there is no assurance that a product will be considered medically reasonable and necessary for a specific indication, will be considered cost-effective by payors, that an adequate level of reimbursement will be established even if coverage is available, or that the payors’ reimbursement policies will not adversely affect the ability for manufacturers to sell products profitably.
Decisions regarding the extent of coverage and reimbursement amount are generally made on a plan-by-plan basis meaning one payor’s decision to cover a particular product does not ensure that other payors will also provide similar coverage. As a result, the coverage determination process can require manufactures to provide scientific and clinical support for the use of a product, and require providers to show medical necessity for use, to each payor separately. This process can be time-consuming, with no assurance that coverage and adequate reimbursement will be applied consistently or even obtained.
Payors are also increasingly reducing reimbursements for devices through continued implementation of cost-containment programs, including price controls and restrictions on coverage and reimbursement, of which could further limit sales of any product. In addition, payors continue to question safety and efficacy while also challenging the prices charged, examining medical necessity and reviewing the cost effectiveness of devices in an effort to avoid coverage and reimbursement. But decreases of this nature surrounding the reimbursement for any product or a decision by a government and third-party payor not to cover a product could result in reduced physician usage and patient demand for the product.
Moreover, in international markets, reimbursement and healthcare payment systems vary significantly by country, with many countries have instituted price ceilings on specific products and therapies.
The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of FDA or another governmental authority, could have a negative impact on us.
We are subject to FDA’s medical device reporting regulations, which require us to report to FDA when we receive or become aware of information that reasonably suggests that one or more of our products may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, it could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event.
We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the initial use of the device. If we fail to comply with our reporting obligations, FDA could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, seizure of our products, or, if premarket review is required in the future, delay in clearance of future products.
| 18 |
| Table of Contents |
FDA and foreign regulatory bodies have the authority to require the recall of commercialized medical device products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects, or other deficiencies or failures to comply with applicable regulations. We cannot assure you that product defects or other errors will not occur in the future. Recalls involving our products could have a material adverse effect on to our business, financial condition, and results of operations.
Moreover, medical device manufacturers are required to maintain certain records of recalls and corrections, even if they are not reportable to FDA. We may initiate voluntary withdrawals or corrections for our devices in the future that we determine do not require notification of FDA. If FDA disagrees with our determinations, then it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability and malpractice claims against us and negatively affect our sales.
We may face difficulties from changes to current regulations and future legislation, both in the U.S. as well as in other foreign jurisdictions where we may be operating.
Existing regulations and regulatory policies may change, and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of our product candidates. Legislative changes may impact our future business and operations, including those that may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our product candidates, if approved, and accordingly, our business, financial condition, and results of operations.
Both before and after a product is commercially released, we have ongoing responsibilities under various laws and regulations. If a regulatory authority were to conclude that we are not in compliance with applicable laws or regulations, or that any of our products are ineffective or pose an unreasonable risk for the end-user, then the authority may ban such devices, detain or seize adulterated or misbranded devices, order a recall, repair, replacement, or refund of such instruments, and require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health. A regulatory authority may also impose operating restrictions, enjoin and restrain certain violations of applicable law pertaining to medical devices, and assess civil or criminal penalties against our officers, employees, or us. The regulatory authority may also recommend prosecution by law enforcement agencies. Any governmental law or regulation, existing or imposed in the future, or enforcement action taken may have a material adverse effect on our business, financial condition, and results of operations.
We cannot predict the likelihood, nature, or extent of any legislative changes will be enacted or government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. Similarly, we cannot predict whether FDA regulations, guidance, or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, then we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
Our industry is highly competitive and subject to significant or rapid technological change.
Our fields of therapeutic interest is highly competitive and subject to significant and rapid technological change. Accordingly, our success may depend, in part, on our ability to respond quickly to such change through the development and introduction of new products.
If our product candidates are approved by FDA, then potential competitors who seek to introduce similar product candidates may seek to take advantage of a shorter and less costly development program for a product that competes with our products. Our ability to compete successfully against currently existing and future alternatives to our product candidates and systems and competitors who compete directly with us may depend, in part, on our ability to attract and retain skilled scientific and research personnel, develop technologically superior products, develop competitively priced products, obtain patent or other required regulatory approvals for our products, be an early entrant to the market and manufacture, market, and sell our products, independently or through collaborations.
| 19 |
| Table of Contents |
We currently rely upon external resources for many engineering and product development services. If we are unable to secure engineering or product development partners or establish satisfactory engineering and product development capabilities, we may not be able to successfully commercialize our technology.
Our success depends upon our ability to develop products that are accurate and provide solutions for our customers. Achieving the desired results for our customers requires solving engineering issues in concert with them. Any failure of our technology or related products to meet customer expectations could result in customers choosing to retain their existing methods or to adopt systems other than ours.
Historically, we have not had sufficient internal resources to work on all necessary engineering and product development matters. We have used third parties in the past and will continue to do so. These resources are not always readily available, and the absence of their availability could inhibit our research and development efforts and our responsiveness to our customers. Our inability to secure those resources could impact our ability to provide engineering and product development services and could have an impact on our customers’ willingness to use our technology. Moreover, third parties have their own internal demands on time and resources which may not always align with ours. Hence, our own expectations for development and product timelines may not be shared by third parties upon whom we rely.
We are in the early stages of commercialization and our technology and related devices may never achieve significant commercial market acceptance.
Our success depends on our ability to develop and market devices that are recognized as accurate, safe and cost-effective. They must be safe and deliver the required level of accuracy under any condition, regardless of the user, as determined by their intended use. This will be achieved through continue refinement of our technology. Before presenting it to the FDA, additional development is needed to increase its generalizability.
Many of our potential customers may be reluctant to use our new technology. Market acceptance will depend on many factors, including our ability to convince potential customers that our technology and related products are an attractive alternative to existing technologies. We will need to demonstrate that our products provide accurate and cost-effective alternatives to existing technologies. Compared to most competing technologies, our technology is new, and most potential customers will have limited knowledge of, or experience with, our products. Prior to implementing our technology and related products, some potential customers may be required to devote significant time and effort to testing and validating our products. Any failure of our technology or related products to meet customer expectations could result in customers choosing to retain their existing methods or to adopt systems other than ours.
Many factors influence the perception of a new technology including its use by leaders in the industry. If we are unable to induce industry leaders in our target markets to implement and use our technology and related products, acceptance and adoption of our products could be slowed. In addition, if our products fail to gain significant acceptance in the marketplace and we are unable to expand our customer base, we may never generate sufficient revenue to achieve or sustain profitability.
Additionally, we may not be able to penetrate or successfully operate in international markets or encounter difficulty expanding into international markets because of limited brand recognition in certain parts of the world, which may lead to delayed acceptance of our products by consumers in these international markets. If we are unable to expand internationally and manage the complexity of international operations successfully, then it could have a material adverse effect on our business, financial condition, and results of operations. If our efforts to introduce our products into foreign markets are not successful, then we may have expended significant resources without realizing the expected benefit. Ultimately, the investment required for expansion into foreign markets could exceed the results of operations generated from this expansion.
| 20 |
| Table of Contents |
We are dependent on key personnel.
Our success depends to a significant degree upon the continued contributions of key management and other personnel, some of whom could be difficult to replace. While our continued operation and ultimate success is not dependent upon one individual, our success does depend on the performance of our officers, our ability to retain and motivate our officers, our ability to integrate new officers into our operations, and the ability of all personnel to work together effectively as a team. Our failure to retain and recruit officers and other key personnel could have a material adverse effect on our business, financial condition and results of operations. Our success also depends on our continued ability to identify, attract, hire, train, retain and motivate highly skilled technical, managerial, manufacturing, administrative and sales and marketing personnel. Competition for these individuals is intense, and we may not be able to successfully recruit, assimilate or retain sufficiently qualified personnel. In particular, we may encounter difficulties in recruiting and retaining a sufficient number of qualified technical personnel, which could harm our ability to develop new products and adversely impact our relationships with existing and future customers. The inability to attract and retain necessary technical, managerial, manufacturing, administrative and sales and marketing personnel could harm our ability to obtain new customers and develop new products and could adversely affect our business and operating results.
We rely on the timely supply of components and parts and could suffer if suppliers fail to meet their delivery obligations, raise prices or cease to supply us with components or parts.
The manufacture of our products is complex and requires the integration of a number of components from several sources of supply. We rely on numerous critical suppliers for various key components that are used in the manufacturing of our products. We can make no assurance that we will be able to maintain such supply arrangements. If we are unable to maintain supply arrangements, our access to key components could be reduced, which could harm our business.
Additionally, if demand for our products decreases, we may have excess inventory and inventory that may expire, which could result in inventory write-offs that would have a material adverse effect on our business, financial condition, and results of operations. We may also encounter defects in materials and/or workmanship, which could lead to a failure to adhere to regulatory requirements. Any defects could delay operations at our contract manufacturers’ facilities, lead to regulatory fines, or halt or discontinue manufacturing indefinitely. Any of these outcomes could have a material adverse effect on our business, financial condition, and results of operations.
This reliance also adds additional risks to the manufacturing process that are beyond our control. For example, the occurrence of epidemics or pandemics may cause one or more of our suppliers to close or reduce the scope of their operations either temporarily or permanently. In addition, these suppliers may provide components and products to our competitors. The medical device industry’s reliance on a limited number of key components and product suppliers subjects us to the risk that in the event of an increase in demand, our suppliers may fail to provide supplies to us in a timely manner while they continue to supply our competitors, many of which have greater purchasing power than us, or seek to supply components to us at a higher cost.
The failure of our suppliers to deliver components or products in a timely fashion could have disruptive effects on our ability to produce our products in a timely manner, or we may be required to find new suppliers at an increased cost.
Moreover, our reputation and the quality of our products are in part dependent on the quality of the components that we source from third-party suppliers. If we are unable to control the quality of the components supplied to us or to address known quality problems in a timely manner, then our reputation in the market may be damaged and sales of our products may suffer. As a result, we may experience a material adverse effect on our business, financial condition, and results of operations.
We have limited insurance which may not cover claims by third parties against us or our officers and directors.
We have directors’ and officers’ liability insurance and commercial liability insurance policies. Claims, however, by third parties against us may exceed policy amounts and we may not have amounts to cover these claims. Any significant claims would have a material adverse effect on our business, financial condition and results of operations. In addition, our limited directors’ and officers’ liability insurance may affect our ability to attract and retain directors and officers.
| 21 |
| Table of Contents |
Our inability to effectively protect our intellectual property would adversely affect our ability to compete effectively, our revenue, our financial condition and our results of operations.
We rely on a combination of patent, trademark, and trade secret laws, and confidentiality procedures to protect our intellectual property rights. Creating and maintaining a strong patent portfolio is important to our business. Patent law relating to the scope of claims in the technology fields in which we operate is complex and uncertain, so we cannot be assured that we will be able to obtain or maintain patent rights, or that the patent rights we may obtain will be valuable, provide an effective barrier to competitors or otherwise provide competitive advantages. Others have filed, and in the future are likely to file, patent applications that are similar or identical to ours or those of our licensors. To determine the priority of inventions or demonstrate that we did not derive our invention from another, we may have to participate in interference or derivation proceedings in the United States Patent and Trademark Office or in court that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. We cannot be assured our patent applications will prevail over those filed by others. Also, our intellectual property rights may be subject to other challenges by third parties. Patents we obtain could be challenged in litigation or in administrative proceedings such as ex parte reexam, inter parties review, or post grant review in the United States or opposition proceedings in Europe or other jurisdictions.
There can be no assurance that:
|
| · | any of our existing patents will continue to be held valid, if challenged; |
|
|
|
|
|
| · | patents will be issued for any of our pending applications; |
|
|
|
|
|
| · | any claims allowed from existing or pending patents will have sufficient scope or strength to protect us; |
|
|
|
|
|
| · | our patents will be issued in the primary countries where our products are sold in order to protect our rights and potential commercial advantage; or |
|
|
|
|
|
| · | any of our products or technologies will not infringe on the patents of other companies. |
If we are prevented from selling our products, or if we are required to develop new technologies or pay significant monetary damages or are required to make substantial royalty payments, our business and results of operations would be harmed.
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or interferences against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could have a material adverse effect on our results of operations and business.
| 22 |
| Table of Contents |
Claims by others that our products infringe their patents or other intellectual property rights could prevent us from manufacturing and selling some of our products or require us to pay royalties or incur substantial costs from litigation or development of non-infringing technology.
In recent years, there has been significant litigation in the United States involving patents and other intellectual property rights. We may receive notices that claim we have infringed upon the intellectual property of others. Even if these claims are not valid, they could subject us to significant costs. Any such claims, with or without merit, could be time-consuming to defend, result in costly litigation, divert our attention and resources, cause product shipment delays or require us to enter into royalty or licensing agreements. Such royalty or licensing agreements, if required, may not be available on terms acceptable to us or at all. We have not been engaged in litigation but litigation may be necessary in the future to enforce our intellectual property rights or to determine the validity and scope of the proprietary rights of others. Litigation may also be necessary to defend against claims of infringement or invalidity by others. A successful claim of intellectual property infringement against us and our failure or inability to license the infringed technology or develop or license technology with comparable functionality could have a material adverse effect on our business, financial condition and operating results.
The analysis of our patent portfolio by PatSnap Research and ipCapital Group is not a legal analysis and does not predict the outcome of any legal challenges we or others might make in regard to patents, nor does it constitute a view on the overall legal strength of our patents.
If we are unable to secure a sales and marketing partner or establish satisfactory sales and marketing capabilities at our company, we may not be able to successfully commercialize our technology.
If we are not successful entering into appropriate collaboration arrangements or recruiting sales and marketing personnel or in building a sales and marketing infrastructure, we will have difficulty successfully commercializing our technology, which would adversely affect our business, operating results and financial condition.
We may not be able to enter into collaboration agreements on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. If we elect to establish a sales and marketing infrastructure, we may not realize a positive return on this investment. In addition, we must compete with established and well-funded pharmaceutical and biotechnology companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize technology without strategic partners or licensees include:
|
| · | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
|
|
|
|
|
| · | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
|
|
|
|
|
| · | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
We may engage in acquisitions, mergers, strategic alliances, joint ventures and divestures that could result in final results that are different than expected.
In the normal course of business, we engage in discussions relating to possible acquisitions, equity investments, mergers, strategic alliances, joint ventures and divestitures. Such transactions are accompanied by a number of risks, including the use of significant amounts of cash, potentially dilutive issuances of equity securities, incurrence of debt on potentially unfavorable terms as well as impairment expenses related to goodwill and amortization expenses related to other intangible assets, the possibility that we may pay too much cash or issue too many of our shares as the purchase price for an acquisition relative to the economic benefits that we ultimately derive from such acquisition, and various potential difficulties involved in integrating acquired businesses into our operations.
From time to time, we have also engaged in discussions with candidates regarding the potential acquisitions of our product lines, technologies and businesses. If a divestiture such as this does occur, we cannot be certain that our business, operating results and financial condition will not be materially and adversely affected. A successful divestiture depends on various factors, including our ability to effectively transfer liabilities, contracts, facilities and employees to any purchaser; identify and separate the intellectual property to be divested from the intellectual property that we wish to retain; reduce fixed costs previously associated with the divested assets or business; and collect the proceeds from any divestitures.
If we do not realize the expected benefits of any acquisition or divestiture transaction, our financial position, results of operations, cash flows and stock price could be negatively impacted.
| 23 |
| Table of Contents |
We may make strategic acquisitions in the future, and if the acquired companies do not perform as expected, this could adversely affect our operating results, financial condition and existing business.
We may continue to expand our business through strategic acquisitions. The success of any acquisition will depend on, among other things:
|
| · | the availability of suitable candidates; |
|
|
|
|
|
| · | higher than anticipated acquisition costs and expenses; |
|
|
|
|
|
| · | competition from other companies for the purchase of available candidates; |
|
|
|
|
|
| · | our ability to value those candidates accurately and negotiate favorable terms for those acquisitions; |
|
|
|
|
|
| · | the availability of funds to finance acquisitions and obtaining any consents necessary under our credit facility; |
|
|
|
|
|
| · | the ability to establish new informational, operational and financial systems to meet the needs of our business; |
|
|
|
|
|
| · | the ability to achieve anticipated synergies, including with respect to complementary products or services; and |
|
|
|
|
|
| · | the availability of management resources to oversee the integration and operation of the acquired businesses. |
We may not be successful in effectively integrating acquired businesses and completing acquisitions in the future. We also may incur substantial expenses and devote significant management time and resources in seeking to complete acquisitions. Acquired businesses may fail to meet our performance expectations. If we do not achieve the anticipated benefits of an acquisition as rapidly as expected, or at all, investors or analysts may not perceive the same benefits of the acquisition as we do. If these risks materialize, our stock price could be materially adversely affected.
Government regulatory approval may be necessary before some of our products can be sold and there is no assurance such approval will be granted.
Our technology will have a number of potential applications in fields of use that will require prior governmental regulatory approval before the technology can be introduced to the marketplace. For example, we are exploring the use of our technology for certain medical diagnostic applications, with an initial focus on the monitoring of blood glucose. There is no assurance that we will be successful in developing glucose monitoring medical applications for our technology. If we were to be successful in developing glucose monitoring medical applications of our technology, prior clearance by FDA and other governmental regulatory bodies will be required before the technology could be introduced into the marketplace. Our devices leverage Machine Learning (ML) and Artificial Intelligence (AI) to process the massive data collected through the Bio-RFID sensor. ML/AI also controls the sensor operation, enabling the device to emit and capture data, and, ultimately, to identify and measure blood glucose levels. Machine learning-enabled device software functions (ML-DSF) continue to be evaluated by FDA, which recently released new guidance proposing a science-based approach for AI/ML-enabled medical devices to be modified and improved more quickly. There is no assurance that such regulatory approval would be obtained for a glucose monitoring medical diagnostic device or other applications requiring such approval. FDA can refuse to grant, delay, and limit or deny approval of an application for clearance of marketing a glucose monitoring device for many reasons. We may not obtain the necessary regulatory approvals or clearances to market these glucose monitoring systems in the United States or outside of the United States. Any delay in, or failure to receive or maintain, approval or clearance for our products could prevent us from generating revenue from these products or achieving profitability.
| 24 |
| Table of Contents |
We or our manufacturers may be unable to obtain or maintain international regulatory clearances or approvals for our current or future products, or our distributors may be unable to obtain necessary qualifications, which could harm our business thus limited sales to the U.S.
Sales of our products internationally are subject to foreign regulatory requirements that vary widely from country to country. In addition, FDA regulates exports of medical devices from the U.S. Complying with international regulatory requirements can be an expensive and time-consuming process, and marketing approval or clearance is not certain. The time required to obtain clearances or approvals, if required by other countries, may be longer than that required for FDA clearance or approvals, and requirements for such clearances or approvals may significantly differ from FDA requirements. We may rely on third-party distributors to obtain regulatory clearances and approvals required in other countries, and these distributors may be unable to obtain or maintain such clearances or approvals. Our distributors may also incur significant costs in attempting to obtain and in maintaining foreign regulatory approvals or clearances, which could increase the difficulty of attracting and retaining qualified distributors. If our distributors experience delays in receiving necessary qualifications, clearances or approvals to market our products outside the U.S., or if they fail to receive those qualifications, clearances or approvals, then we may be unable to market our products or enhancements in international markets effectively, or at all.
Foreign governmental authorities that regulate the manufacture and sale of medical devices have become increasingly stringent and, to the extent we market and sell our products outside of the U.S., we may be subject to rigorous international regulation in the future. In these circumstances, we would be required to rely on our foreign independent distributors to comply with the varying regulations, and any failures on their part could result in restrictions on the sale of our product in foreign countries.
Cybersecurity risks and cyber incidents could result in the compromise of confidential data or critical data systems and give rise to potential harm to customers, remediation and other expenses, expose us to liability under consumer protection laws, or other common law theories, subject us to litigation and federal and state governmental inquiries, damage our reputation, and otherwise be disruptive to our business and operations.
Cyber incidents can result from deliberate attacks or unintentional events. We collect and store on our networks sensitive information, including intellectual property, proprietary business information and personally identifiable information of our customers. The secure maintenance of this information and technology is critical to our business operations. We have implemented multiple layers of security measures to protect the confidentiality, integrity and availability of this data and the systems and devices that store and transmit such data. We utilize current security technologies, and our defenses are monitored and routinely tested internally and by external parties. Despite these efforts, threats from malicious persons and groups, new vulnerabilities and advanced new attacks against information systems create risk of cybersecurity incidents. These incidents can include, but are not limited to, gaining unauthorized access to digital systems for purposes of misappropriating assets or sensitive information, corrupting data, or causing operational disruption. Because the techniques used to obtain unauthorized access, disable or degrade service, or sabotage systems change frequently and may not immediately produce signs of intrusion, we may be unable to anticipate these incidents or techniques, timely discover them, or implement adequate preventative measures.
These threats can come from a variety of sources, ranging in sophistication from an individual hacker to malfeasance by employees, consultants or other service providers to state-sponsored attacks. Cyber threats may be generic, or they may be custom crafted against our information systems. Over the past several years, cyber-attacks have become more prevalent and much harder to detect and defend against. Our network and storage applications may be vulnerable to cyber-attack, malicious intrusion, malfeasance, loss of data privacy or other significant disruption and may be subject to unauthorized access by hackers, employees, consultants or other service providers. In addition, hardware, software or applications we develop or procure from third parties may contain defects in design or manufacture or other problems that could unexpectedly compromise information security. Unauthorized parties may also attempt to gain access to our systems or facilities through fraud, trickery or other forms of deceiving our employees, contractors and temporary staff.
There can be no assurance that we will not be subject to cybersecurity incidents that bypass our security measures, impact the integrity, availability or privacy of personal health information or other data subject to privacy laws or disrupt our information systems, devices or business, including our ability to deliver services to our customers. As a result, cybersecurity, physical security and the continued development and enhancement of our controls, processes and practices designed to protect our enterprise, information systems and data from attack, damage or unauthorized access remain a priority for us. As cyber threats continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any cybersecurity vulnerabilities.
| 25 |
| Table of Contents |
Additionally, the U.S. may institute additional cybersecurity requirements especially for medical devices. For example, the data security requirements in the Food and Drug Omnibus Reform Act (“FDORA”), enacted in December 2022, that among other provisions, requires developers of certain “cyber devices” to design and implement plans to monitor, identify and address cybersecurity vulnerabilities of those devices and to submit those plans to FDA as part of every new 510(k) or PMA for a cyber device. “Cyber devices” are defined as devices that include software, connect to the internet, and contain any technological features that could be vulnerable to cybersecurity threats. This provision entered into effect on March 29, 2023, and FDA has indicated that it expects sponsors of cyber devices to begin to comply with these requirements as of October 1, 2023. FDA has stated that failure to comply with these requirements will result in FDA denying approval of the cyber device application.
We are subject to corporate governance and internal control requirements, and our costs related to compliance with, or our failure to comply with existing and future requirements could adversely affect our business.
We must comply with corporate governance requirements under the Sarbanes-Oxley Act of 2002 and the Dodd–Frank Wall Street Reform and Consumer Protection Act of 2010, as well as additional rules and regulations currently in place and that may be subsequently adopted by the Securities and Exchange Commission, or the SEC, and the Public Company Accounting Oversight Board. These laws, rules, and regulations continue to evolve and may become increasingly stringent in the future. The financial cost of compliance with these laws, rules, and regulations is expected to remain substantial.
We cannot assure you that we will be able to fully comply with these laws, rules, and regulations that address corporate governance, internal control reporting, and similar matters in the future. Failure to comply with these laws, rules and regulations could materially adversely affect our reputation, financial condition, and the value of our securities.
Risks Related to this Offering and Ownership of Our Common Stock
If we are unable to comply with the continued listing requirements of the NYSE American, then our common stock would be delisted from the NYSE American, which would limit investors’ ability to effect transactions in our common stock and subject us to additional trading restrictions.
Our common stock is currently listed on the NYSE American and the continued listing of our common stock on the NYSE American is contingent on our continued compliance with a number of listing requirements. If we are unable to comply with the continued listing requirements of the NYSE American, our common stock would be delisted from the NYSE American, which would limit investors’ ability to effect transactions in our common stock and subject us to additional trading restrictions. In order to maintain our listing, we must maintain certain share prices, financial and share distribution targets, including maintaining a minimum amount of stockholders’ equity and a minimum number of public stockholders, as well as satisfy other listing requirements of the NYSE American. In addition to these objective standards, NYSE American may delist the securities of any issuer for other reasons involving the judgment of NYSE American.
We have been informally advised by the staff of NYSE American that, given our current stockholders equity and history of net losses, we may be subject to the equity standards set forth in Section 1003(a)(ii) and (iii) of the NYSE American Company Guide, and that we may not satisfy these standards or the exemption criteria for these standards. There is no assurance that we will be able to maintain compliance with the NYSE American continued listing rules and/or continue its listing on the NYSE American in the future.
If the NYSE American delists our common stock from trading on its exchange and we are not able to list our securities on another national securities exchange, we expect the common stock would qualify to be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including:
| · | a limited availability of market quotations for our securities; |
|
|
|
| · | reduced liquidity for our securities; |
|
|
|
| · | substantially impair our ability to raise additional funds; |
|
|
|
| · | result in a loss of institutional investor interest and a decreased ability to issue additional securities or obtain additional financing in the future; |
| 26 |
| Table of Contents |
| · | a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
|
|
|
| · | a limited amount of news and analyst coverage; and |
|
|
|
| · | potential breaches of representations or covenants of our agreements pursuant to which we made representations or covenants relating to our compliance with applicable listing requirements, which, regardless of merit, could result in costly litigation, significant liabilities and diversion of our management’s time and attention and could have a material adverse effect on our financial condition, business and results of operations. |
The price of our common stock is volatile, which may cause investment losses for our stockholders.
The market price of our common stock has been and is likely in the future to be volatile. Our common stock price may fluctuate in response to factors such as:
| · | Announcements by us regarding liquidity, significant acquisitions, equity investments and divestitures, strategic relationships, addition or loss of significant customers and contracts, capital expenditure commitments and litigation; |
|
|
|
| · | Issuance of convertible or equity securities and related warrants for general or merger and acquisition purposes; |
|
|
|
| · | Issuance or repayment of debt, accounts payable or convertible debt for general or merger and acquisition purposes; |
|
|
|
| · | Sale of a significant number of shares of our common stock by stockholders; |
|
|
|
| · | General market and economic conditions; |
|
|
|
| · | Quarterly variations in our operating results; |
|
|
|
| · | Investor and public relation activities; |
|
|
|
| · | Announcements of technological innovations; |
|
|
|
| · | New product introductions by us or our competitors; |
|
|
|
| · | Competitive activities; |
|
|
|
| · | Low liquidity; and |
|
|
|
| · | Additions or departures of key personnel. |
These broad market and industry factors may have a material adverse effect on the market price of our common stock, regardless of our actual operating performance. These factors could have a material adverse effect on our business, financial condition, and results of operations.
| 27 |
| Table of Contents |
The sale of a significant number of our shares of common stock could depress the price of our common stock.
As of June 30, 2023, we had 52,358,463 shares of common stock issued and outstanding. As of June 30, 2023, there were options outstanding for the purchase of 14,506,158 shares of our common stock (including unearned stock option grants totaling 3,869,825 shares related to performance targets), warrants for the purchase of 18,856,313 shares of our common stock, 8,108,356 shares of our common stock issuable, collectively, upon the conversion of our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock, and approximately 2,920,000 shares of our common stock, collectively, reserved to pay accrued dividends on our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock. In addition, we currently have 9,020,264 shares of our common stock at the current price of $0.25 per share reserved and are issuable upon conversion of convertible debentures of $2,255,066. Further, under the current terms of our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock, and assuming no changes in the ownership thereof, going forward on a quarterly basis the Company will accrete as a preferred dividend the value of approximately 160,000 shares of common stock, which are issuable if such dividends become payable as additional shares of preferred stock, and such preferred stock is then converted into common stock. All of the foregoing shares could potentially dilute future earnings per share but are excluded from the June 30, 2023, calculation of net loss per share because their impact is antidilutive..
Significant shares of common stock are held by our principal stockholders, other company insiders and other large stockholders. As “affiliates,” as defined under Rule 144 under the Securities Act, our principal stockholders, other of our insiders and other large stockholders may only sell their shares of common stock in the public market pursuant to an effective registration statement or in compliance with Rule 144.
These options, warrants, convertible notes payable and convertible preferred stock could result in further dilution to common stockholders and may affect the market price of the common stock.
Future capital raises or other issuances of equity or debt securities may dilute our existing stockholders’ ownership and/or have other adverse effects on our operations.
Pursuant to our articles of incorporation, we are authorized to issue 200,000,000 shares of common stock. To the extent that common stock is available for issuance, subject to compliance with applicable stock exchange listing rules, our board of directors has the ability to issue additional shares of common stock in the future for such consideration as the board of directors may consider sufficient. The issuance of any additional shares could, among other things, result in substantial dilution of the percentage ownership of our stockholders at the time of issuance, result in substantial dilution of our earnings per share and adversely affect the prevailing market price for our common stock.
Pursuant to our articles of incorporation, we are also authorized to issue 5,000,000 shares of blank check preferred stock of which 30,000 shares have been designated as our Series C Convertible Preferred Stock and 20,000 shares have been designated as our Series D Convertible Preferred Stock. Such preferred stock is senior to our common stock in terms of dividend priority and liquidation preference. Any preferred stock that we issue in the future may rank ahead of our common stock in terms of dividend priority or liquidation preference and may have greater voting rights than our common stock. In addition, such preferred stock may contain provisions allowing those shares to be converted into shares of common stock, which could dilute the value of our common stock to current stockholders and could adversely affect the market price, if any, of our common stock. In addition, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of our company. Although we have no present intention to designate or issue any shares of our authorized blank check preferred stock, there can be no assurance that we will not do so in the future.
As a result of the modifications of our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock (see Description of Securities—Preferred Stock), assuming no changes in the amount of outstanding Preferred Series C or D ownership, going forward on a quarterly basis the Company will accrete as a preferred dividend the value of approximately 160,000 shares of common stock, which are issuable if such dividends become payable as additional shares of preferred stock, and such preferred stock is then converted into common stock.
In the future, we may also attempt to increase our capital resources by offering debt securities. These debt securities would have rights senior to those of our common stock and the terms of the debt securities issued could impose significant restrictions on our operations, including liens on our assets.
| 28 |
| Table of Contents |
Because our decision to issue securities or incur debt in our future offerings will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings and debt financing. Further, market conditions could require us to accept less favorable terms for the issuance of our securities in the future. Thus, you will bear the risk of our future offerings reducing the value of your shares and diluting your interest in us.
The exercise prices of certain warrants, and the conversion prices of our outstanding convertible notes payable and our preferred stock may require further adjustment.
If in the future, we sell our common stock at a price below $0.25 per share, the conversion price of our outstanding shares of Series C Convertible Preferred Stock and Series D Convertible Preferred Stock would adjust below $0.25 per share pursuant to their respective certificates of designation. In addition, the conversion price of the convertible promissory notes referred to above and the exercise price of certain outstanding warrants to purchase 7,684,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments. Warrants totaling 4,439,707 would adjust below $1.20 per share and warrants totaling 4,424,425 would adjust below $2.40 per share, in each case pursuant to the documents governing such instruments.
If our company were to dissolve or wind-up operations, holders of our common stock would not receive a liquidation preference.
If we were to wind-up or dissolve our company and liquidate and distribute our assets, our common stockholders would share in our assets only after we satisfy any amounts we owe to our creditors and preferred equity holders. If our liquidation or dissolution were attributable to our inability to profitably operate our business, then it is likely that we would have material liabilities at the time of liquidation or dissolution. Accordingly, it is very unlikely that sufficient assets will remain available after the payment of our creditors and preferred equity holders to enable common stockholders to receive any liquidation distribution with respect to any common stock.
| 29 |
| Table of Contents |
We estimate that the net proceeds from this offering will be approximately $ , or $ if the underwriters exercise their option to purchase additional shares of common stock assuming a public offering price of $ per share of common stock, the last reported sale price per share of our common stock on September , 2023 as reported on the NYSE American, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The public offering price will be determined between us and the underwriters based on market conditions at the time of pricing and may be at a discount to our current market price. We intend to use the proceeds from this offering for product development, intellectual property development, marketing, operating expenses and general corporate purposes.
Each $0.__ increase or decrease in the assumed public offering price of $ per share of common stock would increase or decrease the net proceeds from this offering by $ , assuming the number of shares, as set forth on the cover of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase or decrease of in the number of shares offered by us, as set forth on the cover of this prospectus, would increase or decrease the net proceeds from this offering by approximately $ , assuming the assumed public offering price per share of common stock remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We do not expect that a change by these amounts in either the public offering price per share of common stock offered by us would have a material effect on our use of the proceeds from this offering, although it may accelerate the time at which we will need to seek additional capital.
We plan to use the net proceeds of this offering as follows:
(dollars in thousands)
|
|
| $ |
|
|
| Product development |
| $ | 2,500 |
|
| Clinical studies |
|
| 2,500 |
|
| General and administrative, intellectual property |
|
| 3,000 |
|
| Working capital |
|
| 2,000 |
|
|
|
| $ | 10,000 |
|
The foregoing represents our current intentions to use and allocate the net proceeds of this offering based upon our present plans and business conditions. Our management, however, will have broad discretion in the way that we use the net proceeds of this offering. Pending the final application of the net proceeds of this offering, we intend to invest the net proceeds of this offering in short‑term, interest‑bearing, investment‑grade securities.
| 30 |
| Table of Contents |
We have never declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any cash dividends on our common stock in the near future. We may also enter into credit agreements or other borrowing arrangements in the future that will restrict our ability to declare or pay cash dividends on our common stock. Any future determination to declare dividends will be made at the discretion of our board of directors subject to limitations under applicable law (including Nevada Revised Statutes 78.288) and will depend on our financial condition, operating results, capital requirements, contractual restrictions, general business conditions and other factors that our board of directors may deem relevant. See also “Risk Factors‑Risks Related to This Offering and Ownership of Our Common Stock”
Our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock do not accrue or pay cash dividends. All future dividends will be accrued and paid in Series C Convertible Preferred Stock or Series D Convertible Preferred Stock, as applicable. See “Description of Securities—Preferred Stock.”
| 31 |
| Table of Contents |
The following table sets forth our capitalization as of June 30, 2023 on an actual basis; and on an as adjusted basis to give effect to the issuance of shares of common stock in the offering at an assumed public offering price of $ per share of common stock, the last reported sale price per share of our common stock on September , 2023, as reported on the NYSE American, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| In thousands of $ |
|
|
|
|
|
|
||
|
|
| June 30, 2023 |
|
|
|
|
||
|
|
| Actual |
|
| Pro Forma (1) |
|
||
|
|
| (Unaudited) |
|
| (Unaudited) |
|
||
| Cash and cash equivalents |
| $ | 3,929 |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
| $ | 2,255 |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
|
| STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
| Series C Convertible Preferred Stock |
| $ | 2 |
|
| $ | - |
|
| Series D Convertible Preferred Stock |
|
| 1 |
|
|
| - |
|
| Common stock |
|
| 52 |
|
|
| - |
|
| Additional paid in capital |
|
| 119,375 |
|
|
|
|
|
| Accumulated deficit |
|
| (118,715 | ) |
|
| - |
|
| Total stockholders' equity |
| $ | 715 |
|
| $ | - |
|
| Total capitalization |
| $ | 2,970 |
|
| $ | - |
|
The pro forma information below is illustrative only and our capitalization following the completion of this offering is subject to adjustment based on the public offering price of our common stock and other terms of this offering determined at pricing. You should read this table together with our financial statements and the related notes in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, subsequent Quarterly Reports on Form 10-Q for the periods ended December 31, 2022, March 31, 2023, and June 30, 2023 each of which is incorporated herein by reference.
If the underwriters exercise the over‑allotment option in full, each of our as adjusted cash, total stockholders’ equity and total capitalization would be $ , $ and $ , respectively.
The table above excludes the following shares:
| · | 14,506,158 shares of our common stock issuable upon the exercise of options which we granted to our officers, directors, and employees under the 2021 Plan (as defined below) at a weighted average exercise price of $1.546 per share (including unearned stock option grants totaling 3,869,825 shares related to performance milestones); |
|
|
|
| · | 21,952,654 additional shares of our common stock that are reserved for issuance under the 2021 Plan; |
|
|
|
| · | 8,108,356 shares of our common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock and approximately 2,920,000 common shares reserved to pay Series C and D preferred stock dividends, through June 30, 2023; |
|
|
|
| · | 9,020,264 shares of our common stock issuable upon the conversion of convertible debentures; |
|
|
|
| · | 18,856,313 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $1.15 per share; and |
|
|
|
| · | up to ________ shares of our common stock issuable upon exercise of the representative’s warrant issued in connection with this offering. |
| 32 |
| Table of Contents |
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock after this offering. Our net tangible book value as of June 30, 2023 was approximately $715,413, or $0.014 per share of our common stock. Net tangible book value per share is equal to our total tangible assets less our total liabilities, divided by the number of shares of our outstanding common stock.
After giving effect to the sale of shares of our common stock in this offering at the assumed public offering price of $ per share (the last reported sale price of our common stock on the NYSE American on September , 2023), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2023 would have been approximately $ , or $ per share of common stock. This represents an immediate increase in as adjusted net tangible book value of $ per share to our existing stockholders, and an immediate dilution of $ per share to new investors purchasing securities in this offering at the assumed public offering price. The final public offering price will be determined between us, the underwriters in the offering based on market conditions at the time of pricing and may be at a discount to the current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final public offering price.
The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share |
|
|
|
| $ | - |
|
|
| Pro forma net tangible book value per share as of June 30, 2023 |
| $ | 0.014 |
|
|
|
|
|
| Increase in net tangible book value per share attributable to this offering |
| $ | (0.014 | ) |
|
|
|
|
| Pro forma as adjusted net tangible book value per share after this offering |
|
|
|
|
| $ | - |
|
| Amount of dilution in net tangible book value per share to new investors in this offering |
|
|
|
|
| $ | - |
|
A $0.50 increase (decrease) in the assumed public offering price of $ per share (the last reported sale price of our common stock on the NYSE American on September , 2023 would result in an increase in our as adjusted net tangible book value per share after this offering by approximately $ and the dilution per share to new investors purchasing shares in this offering by $ assuming the number of securities offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We may also increase or decrease the number of securities to be issued in this offering. Each increase or decrease of shares offered by us would increase or decrease our as adjusted net tangible book value per share and the dilution per share to new investors purchasing securities in this offering by $ assuming that the assumed public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined between us and the underwriter at pricing.
The foregoing discussion and table do not take into account further dilution to investors in this offering that could occur upon the exercise of outstanding options, convertible preferred stock, convertible notes and warrants.
The discussion and table above are based on 52,358,463 shares of our common stock outstanding as of June 30, 2023, and do not include as of June 30, 2023:
| · | 14,506,158 shares of our common stock issuable upon the exercise of options which we granted to our officers, directors, and employees under the 2021 Plan (as defined below) at a weighted average exercise price of $1.546 per share (including unearned stock option grants totaling 3,869,825 shares related to performance milestones); |
|
|
|
| · | 21,952,654 additional shares of our common stock that are reserved for issuance under the 2021 Plan; |
| 33 |
| Table of Contents |
| · | 8,108,356 shares of our common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock and approximately 2,920,000 common shares reserved to pay Series C and D preferred stock dividends, through June 30, 2023; |
|
|
|
| · | 9,020,264 shares of our common stock issuable upon the conversion of convertible debentures; |
|
|
|
| · | 18,856,313 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $1.15 per share; and |
|
|
|
| · | up to ________ shares of our common stock issuable upon exercise of the representative’s warrant issued in connection with this offering. |
The discussion and table above assume no sale of pre-funded warrants, which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis.
To the extent that our outstanding options or warrants are exercised, new options are issued under our equity incentive plan, or additional shares of our common stock are issued in the future, there may be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
| 34 |
| Table of Contents |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis summarizes the significant factors affecting our operating results, financial condition, liquidity and cash flows of our company as of and for the periods presented below. The following discussion and analysis should be read in conjunction with our financial statements and the related notes thereto included elsewhere in this prospectus. The discussion contains forward-looking statements that are based on the beliefs of management, as well as assumptions made by, and information currently available to, our management. Actual results could differ materially from those discussed in or implied by forward-looking statements as a result of various factors, including those discussed below and elsewhere in this prospectus, particularly in the sections titled “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements.”
RESULTS OF OPERATIONS
Overview
We are focused on the development and commercialization of proprietary sensor technology, which, when paired with our machine learning platform, is capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify and measure the unique “signature” of said materials or analytes. We call this our “Bio-RFID™” sensor technology platform. The first application of our Bio-RFID sensor technology is in a product to non-invasively monitor blood glucose levels. This device will require US Food and Drug Administration (FDA) clearance before entering the market.
On April 30, 2020, we incorporated our wholly owned subsidiary, Particle, Inc. Particle was focused on the development and commercialization of our extensive intellectual property relating to electromagnetic energy outside of the medical diagnostic arena, which remains our company’s singular focus. Since incorporation, Particle was engaged in research and development activities on threaded light bulbs that have a warm white light and can inactivate germs, including bacteria and viruses. Particle is now looking for partners to take this product to market.
On September 17, 2021, we incorporated our wholly owned subsidiary, AI Mind, Inc., for the purpose of identifying and capitalizing on market opportunities for our AI deep learning platform (discussed below). The first activity undertaken by AI Mind was the creation of graphical images expressed as non-fungible tokens, or NFTs, utilizing the AI deep learning platform. During the year ended September 30, 2022, AI Mind, operating our AI deep learning platform, began generating revenue from digital asset sales of NFT’s and had sales of $4,351,000. AI Mind was dissolved on July 25, 2023.
Recent Developments
On January 23, 2023, Phillip A. Bosua resigned from the Board of Directors and from his position as our Chief Executive Officer.
On January 23, 2023, our Board of Directors appointed Ronald P. Erickson, the current Chairman of the Board, to the position of Chief Executive Officer.
On January 27, 2023, we announced the following new officers/transitions: Leo Trautwein, Chief Commercial Officer, and Jessica English, Chief Marketing Officer.
On April 21, 2023, we announced the publication of a peer-reviewed study in Sensors Journal. The manuscript described the proof-of-principle study of Bio-RFID technology that quantified three different analytes in vitro. In the peer-reviewed publication, it was found Bio-RFID achieved 100% accuracy in quantifying these three different analytes in vitro. This study was conducted in collaboration with Mayo Clinic.
On May 5, 2023, we announced the results of a technical feasibility study that was presented at the American Association of Clinical Endocrinology (AACE) Annual Meeting. The study demonstrated that the Bio-RFID sensor can deliver stable, repeatable results in predicting blood glucose concentrations obtained by a reference device.
On June 7, 2023, we revealed the portable Generation 1 prototype for non-invasive glucose monitoring. The Generation 1 prototype is a portable research lab, designed to be a powerful data collection device. This device should allow Know Labs to scale data collection, including testing across more diverse participant populations and scenarios.
| 35 |
| Table of Contents |
On July 26, 2023, we announced the completion of a new study demonstrating that continued algorithm refinement and more high-quality data improved the accuracy of the Bio-RFID sensor technology, resulting in an overall Mean Absolute Relative Difference (MARD) of 11.27%.
On August 9, 2023, the Board authorized the Company to file a series of amendments to the certificates of designation for certain series of our preferred stock, and the restatement of its articles of incorporation, as described below, each of which were filed with the Nevada Secretary of State effective August 11, 2023. Based upon the modified terms and conditions of Series C and D certificates of designation, it was determined that Series C and D preferred dividends need to be accreted going forward. As of June 30, 2023, cumulative unpaid Series C and D totaled approximately $730,000 which converts to approximately 2,920,000 shares of common stock. The value of the 2.9 million shares of common totaled $3,337,494. The Company recorded $3,337,494 in cumulative deemed dividends related to Series C and D Preferred Stock which have not been paid.
In connection with the amendment and restatement of our preferred stock, we effected a reverse split of our outstanding Series C Convertible Preferred Stock and Series D Convertible Preferred Stock by a factor of 1-for-100. No changes were made to the 5 million total shares of “blank-check” preferred stock authorized in our Articles. Prior to such reverse split, there were 1,785,715 and 1,016,014 shares of Series C Convertible Preferred Stock and Series D Convertible Preferred Stock designated and outstanding, respectively. To account for the reverse split, but in order to provide the ability to issue “pay in kind” dividends in lieu of cash dividends, at the time of the reverse split, we designated 30,000 shares of Series C Convertible Preferred Stock and 20,000 shares of Series D Convertible Preferred Stock, of which 17,858 and 10,161 shares were, respectively, outstanding immediately after such reverse split. In order to maintain the economic rights of the Series C Convertible Preferred Stock and Series D Convertible Preferred Stock, the definition of “Stated Value” was multiplied by 100, to offset the reverse split factor.
Principal Factors Affecting Our Financial Performance
Our operating results are primarily affected by the following factors:
|
| · | the ability of our research and development team to produce an FDA clearance quality technology; |
|
|
|
|
|
| · | our ability to recruit and maintain quality personnel with the talent to bring our technology to the market; |
|
|
|
|
|
| · | the production of market ready products that can sustain FDA clearance quality results; |
|
|
|
|
|
| · | the clearance by FDA after their rigorous clinical trial process of our products for the marketplace; |
|
|
|
|
|
| · | the receptivity of the marketplace and the addressable diabetes community to our new non-invasive glucose monitoring technology; and |
|
|
|
|
|
| · | access to sufficient capital to support us until our products achieve FDA clearance and are accepted in the marketplace. |
Segment Reporting
The Financial Accounting Standards Board, or FASB, Accounting Standard Codification, or ASC, Topic 280, Segment Reporting, requires that an enterprise report selected information about reportable segments in its financial reports issued to its stockholders. The Company considers the business to currently have one operating segment: the development of its radio frequency spectroscopy technology with a first focus on non-invasively ascertaining blood glucose levels. Previous segments included (i) Particle, Inc. technology; and (ii) AI Mind, Inc. sales of NFT products. Particle commenced operations in the year ended September 30, 2020. It is now looking for partners to take the product to market. AI Mind commenced operations during the year ended September 30, 2022. AI Mind was dissolved on July 25, 2023.
| 36 |
| Table of Contents |
Results of Operations
The following unaudited table sets forth key components of our results of operations during the nine months ended June 30, 2023 and 2022.
(dollars in thousands)
|
|
| Nine Months Ended June 30, |
|
|||||||||||||
|
|
| 2023 |
|
| 2022 |
|
| $ Variance |
|
| % Variance |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| Revenue- digital asset sales |
| $ | - |
|
| $ | 4,360 |
|
| $ | (4,360 | ) |
|
| -100.0 | % |
| Research and development and operating expenses- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development expenses |
|
| 6,186 |
|
|
| 3,407 |
|
|
| 2,779 |
|
|
| -81.6 | % |
| Selling, general and administrative expenses |
|
| 5,508 |
|
|
| 4,255 |
|
|
| 1,253 |
|
|
| -29.4 | % |
| Selling and transactional costs for digital assets |
|
| - |
|
|
| 3,437 |
|
|
| (3,437 | ) |
|
| 100.0 | % |
| Total research and development and operating expenses |
|
| 11,694 |
|
|
| 11,099 |
|
|
| 595 |
|
|
| -5.4 | % |
| Operating loss |
|
| (11,694 | ) |
|
| (6,739 | ) |
|
| (4,955 | ) |
|
| -73.5 | % |
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest income (expense) |
|
| (275 | ) |
|
| (8,024 | ) |
|
| 7,749 |
|
|
| 96.6 | % |
| Other (expense) income |
|
| (384 | ) |
|
| 262 |
|
|
| (646 | ) |
|
| -246.6 | % |
| Total other (expense), net |
|
| (659 | ) |
|
| (7,762 | ) |
|
| 7,103 |
|
|
| 91.5 | % |
| Loss before income taxes |
|
| (12,353 | ) |
|
| (14,501 | ) |
|
| 2,148 |
|
|
| 14.8 | % |
| Income tax expense |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 0.0 | % |
| Net loss |
| $ | (12,353 | ) |
| $ | (14,501 | ) |
| $ | 2,148 |
|
|
| 14.8 | % |
Revenues. Digital asset sales for the nine months ended June 30, 2023 was $0 as compared to $4,360,000 for the nine months ended June 30, 2022. We do not expect future activity or revenue from that source.
Research and Development Expenses. Research and development expenses for the nine months ended June 30, 2023 increased $2,779,000 to $6,186,000 as compared to $3,407,000 for the nine months ended June 30, 2022. The increase was due to increased personnel, use of consultant, expenditures related to the development of our radio frequency spectroscopy Bio-RFID™ technology and approximately $879,000 of termination cash expenses related to the departure of an executive officer.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the nine months ended June 30, 2023 increased $1,253,000 to $5,508,000 as compared to $4,255,000 for the nine months ended June 30, 2022. The increase primarily was due to (i) an increase of $908,000 in stock based compensation; and (ii) an increase in insurance of $387,000; offset by (iii) a decrease in other expenses of $43,000 . As part of the selling, general and administrative expenses for the nine months ended June 30, 2023 and 2022, we recorded $261,000 and $279,000, respectively, of investor relationship and business development expenses.
Selling and Transactional Costs for Digital Asset Sales. Selling and transactional costs for digital asset sales were $0 for the nine months ended June 30, 2023 as compared to $3,437,000 for the nine months ended June 30, 2022. We do not expect future activity or revenue from that source. Our Artificial Intelligence (AI) deep learning platform generated revenue- digital asset sales of $4,360,000 from Non-Fungible Token (NFT) sales for the nine months ended June 30, 2022.
Other (Expense), Net. Other expense, net for the nine months ended June 30, 2023 was $659,000 as compared to other expense, net of $7,762,000 for the nine months ended June 30, 2023. The other expense, net for the nine months ended June 30, 2023 included (i) interest expense, net of $275,000; and (ii) loss on disposal of assets of $384,000 related to the consolidation of leased offices.
The other expense, net for the nine months ended June 30, 2022 included (i) interest expense of $8,024,000 related to convertible notes payable and the amortization of the beneficial conversion feature and value of warrants issued; and offset by (ii) other income of $262,000 primarily related to the forgiveness of notes payable- PPP loans .
Net Loss. Net loss for the nine months ended June 30, 2023 was $12,353,000 as compared to $14,501,000 for the nine months ended June 30, 2022. The net loss for the nine months ended June 30, 2023 included non-cash expenses of $3,454,000. The non-cash items include (i) depreciation and amortization of $259,000; (ii) loss on disposal of assets of $384,000 related to the consolidation of leased offices; (iii) modification of notes and warrants of $350,000; (ix) stock based compensation- stock options of $2,464,000; and offset by (v) other of $3,000.
| 37 |
| Table of Contents |
The net loss for the nine months ended June 30, 2022 included non-cash expenses of $9,243,000. The non-cash items include (i) depreciation and amortization of $219,000; (ii) issuance of common stock for services and expenses of $153,000; (iii) issuance of common stock warrants for service of $71,000; (iv) stock based compensation- stock options of $1,556,000; (v) interest expense for warrant modification of $244,000; (vi) gain on forgiveness of note payable- PPP loans of $253,000; (vii) amortization of debt discount as interest expense of $7,273,000; and offset by (viii) other of $20,000.
Results of Operations During the Years Ended September 30, 2022 and 2021
The following table sets forth key components of our results of operations during the years ended September 30, 2022 and 2021.
(In thousands of $)
|
|
| Years Ended September 30, |
|
|||||||||||||
|
|
| 2022 |
|
| 2021 |
|
| $ Variance |
|
| % Variance |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| Revenue- digital asset sales |
| $ | 4,360 |
|
| $ | - |
|
| $ | 4,360 |
|
|
| 100.0 | % |
| Research and development and operating expenses- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development expenses |
|
| 5,386 |
|
|
| 3,970 |
|
|
| 1,416 |
|
|
| -35.7 | % |
| Selling, general and administrative expenses |
|
| 8,118 |
|
|
| 6,476 |
|
|
| 1,642 |
|
|
| -25.4 | % |
| Selling and transactional costs for digital assets |
|
| 3,430 |
|
|
| - |
|
|
| 3,430 |
|
|
| -100.0 | % |
| Total research and development and operating expenses |
|
| 16,934 |
|
|
| 10,446 |
|
|
| 6,488 |
|
|
| -62.1 | % |
| Operating loss |
|
| (12,574 | ) |
|
| (10,446 | ) |
|
| (2,128 | ) |
|
| -20.4 | % |
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
|
| (8,019 | ) |
|
| (14,914 | ) |
|
| 6,895 |
|
|
| 46.2 | % |
| Other income |
|
| 522 |
|
|
| - |
|
|
| 522 |
|
|
| 100.0 | % |
| Total other (expense), net |
|
| (7,497 | ) |
|
| (14,914 | ) |
|
| 7,417 |
|
|
| 49.7 | % |
| Loss before income taxes |
|
| (20,071 | ) |
|
| (25,360 | ) |
|
| 5,289 |
|
|
| 20.9 | % |
| Income tax expense |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 0.0 | % |
| Net loss |
| $ | (20,071 | ) |
| $ | (25,360 | ) |
| $ | 5,289 |
|
|
| 20.9 | % |
Revenues. Revenue- digital asset sales for the year ended September 30, 2022 was $4,360,000 as compared to $0 for the year ended September 30, 2021. Our Artificial Intelligence (AI) deep learning platform has generated revenue- digital asset sales of $4,360,000 from Non-Fungible Token (NFT) sales.
Research and Development Expenses. Research and development expenses for the year ended September 30, 2022 increased $1,416,000 to $5,386,000 as compared to $3,970,000 for the year ended September 30, 2021. The increase was due increased personnel, use of consultant and expenditures related to the development of our Bio-RFID™ technology.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the year ended September 30, 2022 increased $1,642,000 to $8,118,000 as compared to $6,476,000 for the year ended September 30, 2021. The increase primarily was due to (i) an increase of $3,393,000 in stock based compensation; offset by (ii) $1,051,000 in decreased Particle expenses; (iii) decrease in compensation expense of $2,096,000 related to warrants issued for services and (iv) other decreases of $700,000. As part of the selling, general and administrative expenses for the year ended September 30, 2022 and 2021, we recorded $380,000 and $613,000, respectively, of investor relationship expenses and business development expenses.
Selling and Transactional Costs for Digital Asset Sales. Selling and transactional costs for digital asset sales were $3,430,000 for the year ended September 30, 2022. Our Artificial Intelligence (AI) deep learning platform has generated revenue- digital asset sales of $4,360,000 from Non-Fungible Token (NFT) sales. Such costs included digital asset conversion loss, consulting, bonus compensation transaction fees, taxes, royalties and other costs.
| 38 |
| Table of Contents |
Other (Expense), Net. Other expense, net for the year ended September 30, 2022 was $7,497,000 as compared to other expense, net of $14,914,000 for the year ended September 30, 2021. The other expense, net for the year ended September 30, 2022 included (i) interest expense of $8,019,000 related to convertible notes payable and the amortization of the beneficial conversion feature and value of warrants issued; and offset by (ii) other income of $522,00 primarily related to the forgiveness of notes payable- PPP loans and other debt.
The other expense, net for the year ended September 30, 2021 included interest expense related to convertible notes payable and the amortization of the beneficial conversion feature and value of warrants issued. During the year ended September 30, 2020, we closed a private placement and received gross proceeds of $14,914,000 in exchange for issuing Subordinated Convertible Notes and Warrants in a private placement to accredited investors, pursuant to a series of substantially identical Securities Purchase Agreements, Common Stock Warrants, and related documents.
Net Loss. Net loss for the year ended September 30, 2022 was $20,071,000 as compared to $25,360,000 for the year ended September 30, 2021. The net loss for the year ended September 30, 2022 included non-cash expenses of $12,142,000. The non-cash items include (i) depreciation and amortization of $321,000; (ii) issuance of common stock for services of $183,000; (iii) issuance of common stock warrants for services of $452,000; (iv) stock based compensation- stock options of $4,422,000; (v) amortization of debt discount as interest expense of $7,273,000; (vi) other of $13,000; offset by (vii) gain on debt settlement of $269,000; and (viii) gain on forgiveness of note payable- PPP loans of $253,000.
The net loss for the year ended September 30, 2021 included non-cash expenses of $17,701,000. The non-cash items include (i) depreciation and amortization of $201,000; (ii) issuance for capital stock for services and expenses of $203,000; (iii) stock based compensation- warrants of $2,547,000; (iv) stock based compensation- stock options of $1,029,000; (v) amortization of debt discount as interest expense of $13,722,000; and offset by (vi) other of $1,000.
Liquidity and Capital Resources During the Nine Months Ended June 30, 2023 and 2022
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by operations, levels of accounts receivable and accounts payable and capital expenditures.
As of June 30, 2023, we had cash and cash equivalents of $3,929,000 and net working capital of approximately $2,463,000 (exclusive of convertible notes payable of $2,255,000). We have experienced net losses since inception. As of June 30, 2023, we had an accumulated deficit of $118,715,000 and net losses in the amount of $12,353,000 and $20,071,000 and $25,360,000 during the nine months ended June 30, 2023 and the years ended September 30, 2022 and 2021, respectively. We incurred non-cash expenses of $3,454,000, $12,142,000, and $17,701,000 during the nine months ended June 30, 2023 and the years ended September 30, 2022 and 2021, respectively.
During the end of the quarter ended June 30, 2023, the Company made some adjustments to its staffing level, and the impact of those adjustments, plus the departure of our chief technology and executive officer, has significantly reduced our monthly burn rate. The Company will further adjust its cost structure if new debt or equity capital is not received. We believe that our cash on hand will be sufficient to fund our operations at least through December 31, 2023.
We have financed our corporate operations and our technology development through the issuance of convertible debentures, the issuance of preferred stock, the sale of common stock and the exercise of warrants. During the remainder of 2023, we expect to raise additional funds through the issuance of preferred stock, convertible debentures or equity.
The proceeds of warrants currently outstanding, which are not expected to be exercised on a cashless basis, may generate potential proceeds of up to approximately $15,682,000. We cannot provide assurance that any of these warrants will be exercised but there can be no guarantee that any portion will be exercised.
| 39 |
| Table of Contents |
Operating Activities
Net cash used in operating activities for the nine months ended June 30, 2023 and 2022 was $8,976,000 and $3,691,000, respectively. The net cash used in operating activities for the nine months ended June 30, 2023 was primarily related to (i) a net loss of $12,353,000; and (ii) working capital changes of $77,000; and offset by (iii) non-cash expenses of $3,454,000. The non-cash items include (iv) depreciation and amortization of $259,000; (v) loss on disposal of assets of $384,000 related to the consolidation of leased offices; (vi) modification of notes and warrants of $350,000; (vii) stock based compensation- stock options of $2,464,000; and offset by (xiii) other of $3,000.
The net cash used in operating activities for the nine months ended June 30, 2022 was primarily related to (i) a net loss of $14,501,000; offset by (ii) working capital changes of $1,567,000 related to Our Artificial Intelligence (AI) Deep Learning Platform has generated initial revenue from Non-Fungible Token (NFT) sales and incurred certain expenses; and (iii) non-cash expenses of $9,243,000. The non-cash items include (iv) depreciation and amortization of $219,000; (v) issuance of common stock for services and expenses of $153,000; (vi) issuance of common stock warrants for service of $71,000; (vii) stock based compensation- stock options of $1,556,000; (viii) interest expense for warrant modification of $244,000; (ix) gain on forgiveness of note payable- PPP loans of $253,000; (x) amortization of debt discount as interest expense of $7,273,000; and offset by (xi) other of $20,000.
Investing Activities
Net cash used in investing activities for the nine months ended June 30, 2023 and 2022 was $81,000 and $844,000, respectively. There amounts were primarily related to the investment in equipment for research and development.
Financing Activities
Net cash provided by financing activities for the nine months ended June 30, 2023 and 2022 was $392,000 and $629,000, respectively. The net cash provided by financing activities for the nine months ended June 30, 2023 was primarily related to (i) proceeds from the issuance of common stock for the exercise of warrants of $5,000; and (ii) proceeds from the issuance of common stock for the exercise of stock option grants of $5,000.
The net cash provided by financing activities for the nine months ended June 30, 2022 was primarily related to (i) proceeds from the issuance of common stock for the exercise of warrants of $794,000; (ii) proceeds from the issuance of common stock for the exercise of stock option grants of $14,000; and offset by the settlement of notes payable- PPP loans of $179,000.
Liquidity and Capital Resources During the Years Ended September 30, 2022 and 2021
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by operations, levels of accounts receivable and accounts payable and capital expenditures.
As of September 30, 2022, we had cash and cash equivalents of $12,594,000 and net working capital of approximately $11,040,000 (exclusive of convertible notes payable). We have experienced net losses since inception. As of September 30, 2022, we had an accumulated deficit of $101,398,000 and net losses in the amount of $20,071,000 and $25,360,000 during the years ended September 30, 2022 and 2021, respectively. We incurred non-cash expenses of $12,142,000, and $17,701,000 during the years ended September 30, 2022 and 2021, respectively.
We believe that our cash on hand will be sufficient to fund our operations through December 31, 2023.
We have financed our corporate operations and our technology development through the issuance of convertible debentures, the issuance of preferred stock, the sale of common stock and the exercise of warrants.
On September 20, 2022, we completed a public offering of our common stock pursuant to which we sold 4,140,000 shares of common stock, at a purchase price of $2.00 per share, for total gross proceeds of $8,280,000. After deducting underwriting commissions and other offering expenses, we received net proceeds of $7,425,000.
On March 15, 2021, we closed private placement for gross proceeds of $14,209,000 in exchange for issuing subordinated convertible notes and warrants to purchase 3,552,250 shares of our common stock in a private placement to accredited investors. These convertible notes were automatically converted into shares of our common stock at a conversion price of $2.00 per share starting on March 9, 2022. The convertible notes had an original principal amount of $14,209,000 with an annual interest of 8%. Both the principal amount and the interest were payable on a payment-in-kind basis in shares of our common stock.
| 40 |
| Table of Contents |
The proceeds of warrants currently outstanding, which are not expected to be exercised on a cashless basis, may generate potential proceeds of up to approximately $15,694,000. We cannot provide assurance that any of these warrants will be exercised.
Operating Activities
Net cash used in operating activities for the year ended September 30, 2022 and 2021 was $6,920,000 and $6,851,000, respectively. The net cash used in operating activities for the year ended September 30, 2022 was primarily related to (i) a net loss of $20,071,000; offset by (ii) working capital changes of $1,009,000 related to Our Artificial Intelligence (AI) Deep Learning Platform has generated initial revenue from Non-Fungible Token (NFT) sales and incurred certain expenses; and (iii) non-cash expenses of $12,142,000. The non-cash items include (iv) depreciation and amortization of $321,000; (v) issuance of common stock for services of $183,000; (vi) issuance of common stock warrants for services of $452,000; (vii) stock based compensation- stock options of $4,422,000; (viii) amortization of debt discount as interest expense of $7,273,000; (ix) other of $13,000; offset by (x) gain on debt settlement of $269,000; and (xi) gain on forgiveness of note payable- PPP loans of $253,000.
The net cash used in operating activities for the year ended September 30, 2021 was primarily related to (i) a net loss of $25,360,000; offset by (ii) working capital changes of $810,000; and (ii) non-cash expenses of $13,050,000. The non-cash items include (iii) depreciation and amortization of $201,000; (iv) issuance for capital stock for services and expenses of $203,000; (v) stock based compensation- warrants of $2,547,000; (vi) stock based compensation- stock options of $1,029,000; (vii) amortization of debt discount as interest expense of $13,722,000; and offset by (viii) other of $1,000.
Investing Activities
Net cash used in investing activities for the year ended September 30, 2022 and 2021 was $855,000 and $300,000, respectively. There amounts were primarily related to the investment in equipment for research and development.
Financing Activities
Net cash provided by financing activities for the year ended September 30, 2022 and 2021 was $8,111,000 and $15,110,000, respectively. The net cash provided by financing activities for the year ended September 30, 2022 was primarily related to (i) proceeds from the issuance of common stock for the exercise of warrants of $838,000; (ii) proceeds from the issuance of common stock for the exercise of stock option grants of $27,000; issuance of common stock for NYSE uplisting, net of expenses of $7,425,000; and offset by the repayment of notes payable- PPP loans of $179,000. On September 20, 2022, we completed a public offering of our common stock pursuant to which we sold 4,140,000 shares of common stock, at a purchase price of $2.00 per share, for total gross proceeds of $8,280,000. After deducting underwriting commissions and other offering expenses, we received net proceeds of $7,425,000.
The net cash provided by financing activities for the year ended September 30, 2021 was primarily related to (i) issuance of Simple Agreements for future Equity of $340,000; (ii) $14,209,000 related to proceeds from convertible notes payable; (iii) proceeds from notes payable- PPP of $206,000; (iv) proceeds from the issuance of common stock for the exercise of warrants of $1,313,000; (v) proceeds from the issuance of common stock for the exercise of stock option grants of $23,000; and offset by (vi) payment of issuance costs from notes payable of $727,000 and (vii) repayments on Simple Agreements for Future Equity.
On March 15, 2021, we closed private placement for gross proceeds of $14,209,000 in exchange for issuing Subordinated Convertible Notes and 3,552,250 Warrants in a private placement to accredited investors, pursuant to a series of substantially identical Securities Purchase Agreements, Common Stock Warrants, and related documents. The Convertible Notes will be automatically converted to our Common Stock at $2.00 per share on the one year anniversary starting on March 15, 2022.
| 41 |
| Table of Contents |
The Convertible Notes had an original principal amount of $14,209,000 and bear annual interest of 8%. Both the principal amount and the interest are payable on a payment-in-kind basis in shares of our Common Stock.
Our contractual cash obligations as of June 30, 2023 are summarized in the table below:
|
|
|
|
| Less Than |
|
|
|
|
|
| Greater Than |
|
||||||||
| Contractual Cash Obligations (1) |
| Total |
|
| 1 Year |
|
| 1-3 Years |
|
| 3-5 Years |
|
| 5 Years |
|
|||||
| Operating leases |
| $ | 206,000 |
|
| $ | 206,000 |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| Convertible notes payable |
|
| 2,255,000 |
|
|
| 2,255,000 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
| $ | 2,461,000 |
|
| $ | 2,461,000 |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
(1) Convertible notes payable includes $2,255,000 that can be converted into common stock upon demand. We expect to incur capital expenditures related to the development of the “Bio-RFID™” and “ChromaID” technologies. None of the expenditures are contractual obligations as of June 30, 2023.
Critical Accounting Policies
The preparation of financial statements in conformity with GAAP requires our management to make assumptions, estimates and judgments that affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial condition and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments. We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements:
Revenue Recognition – We determine revenue recognition from contracts with customers through the following steps:
|
| · | identification of the contract, or contracts, with the customer; |
|
|
|
|
|
| · | identification of the performance obligations in the contract; |
|
|
|
|
|
| · | determination of the transaction price; |
|
|
|
|
|
| · | allocation of the transaction price to the performance obligations in the contract; and |
|
|
|
|
|
| · | recognition of the revenue when, or as, the Company satisfies a performance obligation. |
Revenue is recognized when control of the promised goods or services is transferred to the customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. During the three months ended December 31, 2021, we generated revenue from digital asset sales of NFTs. Our engineering team, using its research data, AI and proprietary algorithms, produced NFTs in the form of digital art. The NFTs produced had no recorded cost basis. The Company does not expect future activity or revenue from that source.
Research and Development Expenses – Research and development expenses consist of the cost of officers, employees, consultants and contractors who design, engineer and develop new products and processes as well as materials, supplies and facilities used in producing prototypes.
Our current research and development efforts are primarily focused on improving its radio frequency spectroscopy technology and its first focus on non-invasive monitoring of blood glucose levels; extending its capacity and developing new and unique applications for this technology. We believe that continued development of new and enhanced technologies is essential to its future success. We incurred expenses of $6,186,039 and $3,406,996 for the nine months ended June 30, 2023, and 2022, respectively, on development activities. Included in the expense for 2023 is approximately $859,000 related to severance and other expenses associated with the departure of the Company’s former chief technology officer and chief executive officer, Philip A. Bosua, and other employees.
| 42 |
| Table of Contents |
Fair Value Measurements and Financial Instruments – ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
Level 1 – Quoted prices in active markets for identical assets and liabilities;
Level 2 – Inputs other than level one inputs that are either directly or indirectly observable; and
Level 3 – Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The recorded value of other financial assets and liabilities, which consist primarily of cash and cash equivalents, accounts receivable, other current assets, accounts payable and accrued expenses approximate the fair value of the respective assets and liabilities as of June 30, 2023 and September 30, 2022 are based upon the short-term nature of the assets and liabilities. The fair value of our convertible notes payable are not readily available given the terms and conditions, including the conversion features, are complex.
We have a money market account which is considered a Level 1 asset. The balance as of June 30, 2023 and September 30, 2022 was $3,678,865 and $11,821,931, respectively. No other assets or liabilities are required to be recorded at fair value on a recurring nature.
Stock Based Compensation – We have share-based compensation plans under which employees, consultants, suppliers and directors may be granted restricted stock, as well as options and warrants to purchase shares of our common stock at the fair market value at the time of grant. Stock-based compensation is measured by the Company at the grant date, based on the fair value of the award, over the requisite service period under ASC 718. We recognizes stock compensation costs utilizing the fair value methodology over the related period of benefit.
Convertible Securities – Based upon ASC 815-15, we have adopted a sequencing approach regarding the application of ASC 815-40 to convertible securities. We will evaluate its contracts based upon the earliest issuance date. In the event partial reclassification of contracts subject to ASC 815-40-25 is necessary, due to our inability to demonstrate it has sufficient shares authorized and unissued, shares will be allocated on the basis of issuance date, with the earliest issuance date receiving first allocation of shares. If a reclassification of an instrument were required, it would result in the instrument issued latest being reclassified first.
Overview
Know Labs is an emerging leader in non-invasive medical diagnostics. We are focused on the development and commercialization of our proprietary sensor technology utilizing radio and microwave spectroscopy. When paired with our machine learning platform, our technology is capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify, and measure the unique “signature” of said materials or analytes. We call this our “Bio-RFID™” sensor technology platform.
The first application of our Bio-RFID sensor technology is in a product to non-invasively monitor blood glucose levels. Our device will provide the user with real-time information on their blood glucose levels. We recently announced our Generation 1 working prototype device. This device embodies the Bio-RFID sensor which has been used in internal clinical testing. We will expand our testing, both internally and externally with the Generation 1 device and will refine the device itself over time into final form factors. These devices will require FDA clearance before entering the market.
| 43 |
| Table of Contents |
Following FDA clearance of our non-invasive blood glucose monitoring device, Know Labs plans to expand Bio-RFID to other non-invasive medical diagnostic applications. As a platform technology, Bio-RFID can identify numerous other analytes in the human body that are important in medical diagnostics and human health and wellness.
While medical diagnostics applications, with blood glucose monitoring paramount, are the focus of Know Labs, the Company’s proprietary radio frequency and microwave spectroscopy platform have broad applicability outside of the medical diagnostic realm. Over time, as resources allow, the Company will explore those opportunities.
Corporate History and Structure
Know Labs, Inc. was incorporated under the laws of the State of Nevada in 1998. Since 2007, our company has been focused primarily on research and development of proprietary spectroscopic technologies spanning the electromagnetic spectrum.
Know Labs has one wholly owned subsidiary, Particle, Inc. incorporated on April 30, 2020. AI Mind, Inc., Know Lab’s former wholly owned subsidiary, was incorporated on September 17, 2021 and dissolved in early 2023. At this time there is no material activity in the Particle subsidiary while the Company gives all of its attention to its focus on its Bio-RFID technology.
The Know Labs Technology
We have internally and under contract with third parties developed proprietary platform technology to uniquely identify and measure almost any organic and inorganic material or analyte. Our patented technology utilizes electromagnetic energy along a wide range of the electromagnetic spectrum from visible light and infrared to radio wave and microwave wavelengths to perform analytics which allow the user to accurately identify and measure materials and analytes.
Our technology provides a unique platform upon which a myriad of applications can be developed. As a platform technology, it is analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. Our radio frequency spectroscopy technology is an “enabling” technology that brings the science of electromagnetic energy to low-cost, real-world commercialization opportunities across multiple industries. The technology is foundational and, as such, the basis upon which we believe significant businesses can be built. While we are pursuing our core focus on commercializing our glucose monitor, we believe non-core clinical, non-clinical and medical research applications represent a multitude of opportunities for strategic collaboration, joint development, and licensing agreements with leading companies in their respective industries.
We believe an important competitive differentiator for Bio-RFID to be its ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so non-invasively, and in real-time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring.
Bio-RFID: Hardware and Software
Our Bio-RFID technology embodies two key components: hardware and software. The key hardware component includes a sensor which both sends and receives a radio frequency signal. The data obtained by the receiving aspect of the sensor is analyzed by software. Today, the sensor portion of our hardware development is complete. This sensor is currently being used in our internal tests, and has been for the past several months, gathering millions of data points to further refine our algorithm. It is the core component in our Generation 1 working prototype device, which we have recently disclosed, and will be the core component of our eventual final marketed product pending FDA clearance.
As a consequence, a significant amount of our focus has shifted to algorithm development. This involves sophisticated development of algorithms which derive meaningful information from the raw data obtained by our sensor. These algorithms are developed through the utilization of artificial intelligence (AI) and machine learning (ML) by means of training varying models. We will continue data collection to further refine the accuracy of the algorithm until we feel confident that we can be successful in FDA clinical trials and bring to the market the first non-invasive blood glucose monitor.
| 44 |
| Table of Contents |
Bio-RFID: Early Results
We previously announced the results of an internal exploratory study comparing tests between our Bio-RFID technology and the leading continuous glucose monitors from Abbott Labs (Freestyle Libre®) and DexCom (G6®). These results provided evidence of a high degree of correlation between our Bio-RFID technology and the current industry leaders and their continuous glucose monitors. Our patented technology is fundamentally differentiated from these industry leaders as our technology completely non-invasively monitors blood glucose levels. We also believe Bio-RFID successfully addresses the limiting qualities of non-invasive optical technologies whose diagnostic capacities may be inhibited by skin tones and other factors.
We continue to build the internal and external development team necessary to commercialize our technology. Our ability to obtain exacting results from the data collected through our Bio-RFID sensor technology, also referred to as radio frequency spectroscopy or RF spectroscopy, is enabled by our trade secret algorithms built through our machine learning platform. We have been refining these algorithms so they can accurately determine blood glucose levels. We believe our algorithms can also provide accurate measurements for blood alcohol and blood oxygen levels, which we have identified in preliminary tests. We expect them to provide the analytics for the long list of other potential analytes in the human body many of which are set forth in our issued patent USPTO 11,033,208 B1.
Bio-RFID: Validation and FDA Clearance
We are also focused on building strong external validation of the Bio-RFID technology. This on-going initiative should provide additional evidence and support as we look to approach FDA. Over the past several months we have announced several significant validating studies. They include: The results of a proof-of-principle study titled, “Detecting Unique Analyte-Specific Radio Frequency Spectral Responses in Liquid Solutions, Implications for Non-Invasive Physiologic Monitoring.” This study was conducted in collaboration with Mayo Clinic, sponsored by the Company, and its results were presented at the 2023 American Physiological Society (APS) Summit. The study demonstrated the accuracy of the Bio-RFID sensor in quantifying
three different analytes in vitro. In the peer-reviewed publication, it was found Bio-RFID achieved 100% accuracy in quantifying these three different analytes in vitro. The study was peer-reviewed by Sensors Journal and American Physiology Society.
The results of our technical feasibility study titled, “Technical Feasibility of a Novel Sensor for Non-Invasive Blood Glucose Monitoring Compared to Dexcom G6®.” These results were presented at the American Association of Clinical Endocrinology (AACE) Annual Meeting in Seattle, WA. The study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle. The purpose of this technical feasibility study was to demonstrate hardware and software infrastructure stability, and to collect additional data to determine the accuracy of the sensor at quantifying BGC in vivo non-invasively using radio frequency by means of training a neural network (NN) model to predict readings of the Dexcom G6® as a proxy for BGC. The study was peer-reviewed by the American Association of Clinical Endocrinology.
The results of a new study titled, "Algorithm Refinement in the Non-Invasive Detection of Blood Glucose Using Know Labs’ Bio-RFID Technology." The study demonstrates that algorithm optimization using a light gradient-boosting machine (lightGBM) machine learning model improved the accuracy of Know Labs’ Bio-RFID™ sensor technology at quantifying blood glucose using predicted readings of the Dexcom G6® as a proxy for BGC, demonstrating an overall Mean Absolute Relative Difference (MARD) of 12.9% – which is within the range of independently reported values for certain FDA-cleared blood glucose monitoring devices. The study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle, and reviewed by members of Know Labs' Scientific Advisory Board.
The results from a new study6 titled, "Novel data preprocessing techniques in an expanded dataset improve machine learning model accuracy for a non-invasive blood glucose monitor." The study demonstrates that continued algorithm refinement and more high-quality data improved the accuracy of Know Labs’ proprietary Bio-RFID sensor technology, resulting in an overall Mean Absolute Relative Difference (MARD) of 11.3%. As with all Know Labs’ previous research, this study was designed to assess the ability of the Bio-RFID sensor to non-invasively and continuously quantify blood glucose, using the Dexcom G6® continuous glucose monitor (CGM) as a reference device and proxy for BGC. In this new study where data collection was completed in May of 2023, Know Labs applied novel data preprocessing techniques and trained a light gradient-boosting machine (lightGBM) model to predict blood glucose values of Dexcom G6® CGM using 3,311 observations – or reference device values – from over 330 hours of data collected from 13 healthy participants. With this method, Know Labs was able to predict blood glucose in the test set – the dataset that provides a blind evaluation of model performance – with a MARD of 11.3%. The study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle, and reviewed by members of Know Labs' Scientific Advisory Board.
| 45 |
| Table of Contents |
As the Company successfully completed our foundational studies, created a stable sensor that delivers repeatable results, and developed a software infrastructure to manage and interpret large, novel datasets, it will continue to expand its testing and data gathering with larger and more diverse populations in order to acquire generalizable results, a core element on the path to FDA clearance.
We have also begun the internal and external process to pursue FDA clearance for our non-invasive blood glucose monitor. Our Chief Medical Officer, medical and regulatory advisory board, our entire executive team along with external advisors guide us in this process. Additionally, our third-party quality assurance and documentation consultants help ensure that the rigorous requirements of FDA are met. We are unable to estimate the time necessary for FDA approval or the likelihood of success in that endeavor.
While the first focus of our Bio-RFID platform is non-invasive glucose monitoring, it is important to note that the Bio-RFID platform has the capacity to monitor and identify other analytes in the human body. Each additional analyte we identify over time may require its own subsequent FDA clearance, the success of which we are unable to estimate at this time. Our radio frequency spectroscopic technology is the foundational platform for the development of these future applications of Bio-RFID.
Product Strategy
We have announced the development of our non-invasive glucose monitor and our desire to obtain FDA clearance for the marketing of this product. We are currently undertaking internal development work of this product for the commercial marketplace. We have also announced the engagement of several strategic partners and advisors focused on sensor technology, product design, data science, machine learning, manufacturing and regulatory affairs, who we will work with to bring this product to market. The recent announcement of our Generation 1 working prototype device was a significant milestone for the Company. It will be used in internal and external clinical testing and will gather significant amounts of data with diverse populations which will allow us to refine the design of the next generation device. We will make further announcements regarding the product as development, testing, manufacturing, and regulatory approval work progresses.
Our efforts are entirely focused on productizing Bio-RFID and collecting high quality data for validation purposes, including third-party studies, and appropriate and required clinical trials. At this point in our development cycle, the hardware continues to be miniaturized and optimized, the product form factor is moving in the direction of a final product that will be used for FDA clinical trials and the algorithms which provide results from the data collected by our sensor are being refined to improve accuracy.
Sales and Marketing
While we continue with our internal development efforts and the move toward clinical trials for FDA clearance and expected (but not guaranteed) clearance of our first product, a non-invasive blood glucose monitor, we will explore the several potential avenues for moving our first product and potential follow-on products into the marketplace. The avenues being explored include direct to consumer, initial launch partners, broad distribution partners, licensing partners and private label approaches to the market among others. We have begun to build our internal commercial and marketing team in preparation for detailed strategic thinking about the optimal approach to the marketplace. We attend and engage in conferences focused on diabetes management and technology, which are valuable for building Know Labs’ reputation and network in the space.
Competition
The technology industry, generally, and blood glucose monitoring and other medical diagnostic markets, in particular, are intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities by industry participants. To compete successfully, we will need to demonstrate the advantages of our products and technologies over well-established alternative solutions, products, and technologies, including legacy providers of blood glucose monitoring technology, as well as newer ones that are working to achieve a non-invasive solution or more acceptable blood glucose monitoring solutions which may or may not be similar to our technology, and convince consumers and enterprises of the advantages of our products and technologies.
| 46 |
| Table of Contents |
We group our competition into three large categories. Those are (i) large global technology companies who may enter the blood glucose monitoring and other medical diagnostic markets, (ii) legacy providers of blood glucose monitoring technology, and (iii) new entrants working to achieve a non-invasive solution or more acceptable blood glucose monitoring solutions which may or may not be similar to our technology. With regard to companies in each category, we perform due diligence from all publicly available sources of information on their relevant technologies and their product plans. This information informs and refines our activities and underscores our sense of urgency as we work to bring our own technology to the marketplace. As it relates to all competitors, we continue to focus on building the world’s most robust patent portfolio in this space. PatSnap Research and ipCapital Group, two leading patent analytic firms, have ranked Know Labs #1 for global patent leadership in non-invasive glucose monitoring patents. We have retained both organizations to perform patent related work. We continue to build out our patent portfolio and grow our trade secret AI and ML driven algorithms.
With respect to our planned non-invasive glucose monitoring solution, we will face direct and indirect competition from a number of competitors who have developed or are developing products for continuous monitoring of glucose levels. These competitors include DexCom, Inc., Abbott Laboratories, Medtronic plc, Roche Diagnostics, LifeScan, Inc., Ascensia Diabetes Care Holdings AG, Senseonics Holdings, Inc., Integrity Applications, Inc., Nemaura Medical, Biolinq Inc., and Profusa, Inc. Our planned solution will also compete with traditional glucometers, which remain an inexpensive alternative. We also compete with companies who are seeking to create non-invasive glucose monitors, such as Movano, Inc. Because of the large size of the potential market for our products, it is possible that new or existing competitors may develop competing products, procedures, or clinical solutions that could prove to be more effective, safer, or less costly than our solution. The introduction of new products, procedures, or clinical solutions by competitors may result in price reductions, reduced margins, or loss of market share, or may render our products obsolete. Many of the companies we will compete with enjoy significantly greater name recognition and have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and sales and marketing of approved products than we have.
Mergers and acquisitions in the medical device, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Other small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. There are also several academic and other institutions involved in various phases of technology development regarding blood glucose monitoring devices.
Competitive Advantages
We believe our key competitive strengths include:
|
| · | Through first principles, Bio-RFID’s ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so non-invasively, and in real time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring. |
|
|
|
|
|
| · | Our Bio-RFID technology is non-invasive, using radio waves to identify and measure what is going on inside the body in real-time. |
|
|
|
|
|
| · | Our Bio-RFID technology platform can be integrated into a variety of wearable, mobile, or counter-top form factors, and we believe interoperability with existing products from current market leaders. |
|
|
|
|
|
| · | No needles nor invasive transmitters in your body, making Bio-RFID sensors convenient and pain-free. |
|
|
|
|
|
| · | No expensive supplies, such as test strips and lancets, are required to operate Bio-RFID devices. |
|
|
|
|
|
| · | A core focus on accessibility and affordability for the populations we will serve around the globe. |
|
|
|
|
|
| · | The current prototype sensor collects approximately 1.5 million data points per hour, which allows Bio-RFID to potentially build a deep understanding of health and wellness that other sensors may not be able to. |
|
|
|
|
|
| · | Know Labs is the world intellectual property leader in non-invasive blood glucose monitoring, according to ipCG Capital and PatSnap, with more than 150 patents issued and pending related to its core-business. |
| 47 |
| Table of Contents |
Growth Strategy
The key elements of our strategy to grow our business include:
|
| · | Initially, entering the diabetes glucose monitoring market with our non-invasive glucose monitoring devices. |
|
|
|
|
|
| · | Following our entry into the glucose monitoring market, entering other clinical monitoring markets for continuous, non-invasive hormone, medication metabolites, endocrinology components, and biomolecular monitoring. |
|
|
|
|
|
| · | Applying our Bio-RFID platform technology to lifestyle analysis, clinical trials, and chronic illnesses. We believe that potential use cases include real-time wearable medication monitoring and detection of, for example, ovulation and hormone deficiency. |
|
|
|
|
|
| · | With an ever-growing body of non-invasively determined analytes available from individuals utilizing our Bio-RFID technology we believe, over time, with longitudinal data we will be able to engage in so-called “predictive health” and provide early warnings of the onset of disease. |
|
|
|
|
|
| · | Significantly, every new application will function utilizing the same sensor. We expect that hardware changes will not be required to target new analytes, so you will not need a new device, but an updated software algorithm will be required. |
|
|
|
|
|
| · | Each new application provides potential new opportunities for monetization of the Bio-RFID platform technology. Each additional analyte we identify over time may require its own subsequent FDA approval. |
Research and Development
Our current research and development efforts are primarily focused on improving our radio frequency spectroscopy Bio-RFID technology for the monitoring of blood glucose. As part of this effort, we continuously perform clinical testing of our devices following IRB-approved protocols, and we conduct on-going laboratory testing to ensure that application methods are compatible with the end-user and regulatory requirements, and that they can be implemented in a cost-effective manner. As resources permit, we plan to focus on extending the capacity of Bio-RFID to identify new analytes and applications. Our current internal team along with outside consultants have considerable experience working with the application of our technologies. We engage third party experts as required to supplement our internal team. We incurred expenses of approximately $6,186,000, $5,386,000 and $3,970,000 for the nine months ended June 30, 2023 and the years ended September 30, 2022 and 2021, respectively, on development activities.
Intellectual Property
The cornerstone of our foundational platform technology is our intellectual property portfolio. We have pursued an active intellectual property strategy which includes focus on patents where appropriate and a diligent protection of trade secrets. To date, we have been granted 31 patents and 19 design patents. These include 12 patents on our early work on the visible and near visible portions of the electromagnetic spectrum, which were a point of creative departure as we explored and invented our Bio-RFID technology. We currently have a number of patents pending and continue, on a regular basis, with the filing of new patents. If we include pending patents, our IP portfolio reaches 169 patents issued and pending, which positions the company as the top worldwide IP holder in non-invasive blood glucose monitoring, according to ipCapital Group, a leading IP and innovation consulting firm. We possess all rights, title and interest to the issued patents.
| 48 |
| Table of Contents |
Our issued patents will expire at various times between 2027 and 2041. Pending patents, if and when issued, may have expiration dates that extend further in time. The duration of our trademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
The issued patents cover the fundamental aspects of our radio frequency spectroscopy technology and a number of unique applications. We have filed patents, which are pending, on the additional fundamental aspects of our technology and growing number of unique applications. We will continue, over time, to expand our patent portfolio.
Additionally, significant aspects of our technology are maintained as trade secrets which may not be disclosed through the patent filing process. We are diligent in maintaining and securing our trade secrets, in particular as they involve our AI and ML driven algorithms.
We shall also have an exclusive, perpetual and royalty free right to any patent(s) or other intellectual property which Phillip Bosua, someone working under direction of Phillip Bosua, or any successor or assignee develops relating to the Bio-RFID technology within a period of five years after January 23, 2023.
Related Patent Assets
Inherent in a platform technology is the ability to develop or license technology in diverse fields of use apart from our core focus. We focus on human health and wellness with a first focus on the non-invasive monitoring of blood glucose. We plan to pursue the identification of a multitude of analytes in the human body that are important to diagnostics over time. We also plan to identify, over time, opportunities for our intellectual property to be deployed in areas outside of human health and wellness.
We may, although we cannot guarantee that we will, create other such subsidiaries over time. Additionally, we may license our intellectual property to third parties so that they may pursue activities that are not a part of our core focus.
Employees
As of June 30, 2023, we had 11 full-time employees. Our senior management and other personnel are primarily located in our Seattle, Washington offices with some hybrid remote work. The Company expanded its utilization of consulting firms and individual contractors to supplement our reduced workforce in an effort to reduce fixed expenses and extend operating resources.
Government Regulation
Our operations are subject to comprehensive federal, state, and local laws and regulations in the jurisdictions in which we or our research and development partners do business. The laws and regulations governing our business and interpretations of those laws and regulations and are subject to frequent change. Our ability to operate profitably will depend in part upon our ability, and that of our research and development partners and affiliates, to operate in compliance with applicable laws and regulations. The laws and regulations relating to medical that apply to our business and that of our partners and affiliates continue to evolve, and we must, therefore, devote significant resources to monitoring developments in legislation, enforcement, and regulation in such areas. As the applicable laws and regulations change, we are likely to make conforming modifications in our business processes from time to time. We cannot provide assurance that a review of our business by courts or regulatory authorities will not result in determinations that could adversely affect our operations or that the regulatory environment will not change in a way that restricts our operations.
United States FDA Regulation
The KnowU and UBand glucose monitoring products will be designed to allow our Bio-RFID™ sensor technology platform to generate a glucose value and provide the user with real-time information on their blood glucose levels. A patient’s glucose data will be displayed on the KnowU and UBand glucose monitoring products and may potentially have capability to be transmitted directly to certain compatible mobile devices, including iPhone®, iPod touch®, iPad®, and Android® devices.
| 49 |
| Table of Contents |
Our medical diagnostic products and operations, initially the KnowU and UBand glucose monitoring products, are subject to extensive and rigorous regulation by the U.S. Food and Drug Administration, or FDA, under the Federal Food, Drug, and Cosmetic Act, or FFDCA, and its implementing regulations, guidance documentation, and standards. Our KnowU and UBand products will be regulated by FDA as medical devices. FDA regulates the design, development, research, testing, manufacturing, safety, labeling, storage, recordkeeping, promotion, distribution, sale and advertising of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. FDA also regulates the export of medical devices manufactured in the United States to international markets. Any violations of these laws and regulations could result in a material adverse effect on our business, financial condition and results of operations. In addition, if there is a change in law, regulation or judicial interpretation, we may be required to change our business practices, which could have a material adverse effect on our business, financial condition and results of operations.
Under the FFDCA, medical devices are generally classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk to the patient and/or the user associated with each medical device and the extent of control needed to ensure safety and effectiveness. Device classification also depends on the intended use of the device and upon indications for use. Additionally, the class to which your device is assigned also determines, among other things, the type of premarketing submission/application required for FDA clearance to market.
Class I includes devices with the lowest risk, present a minimal potential for harm and for which safety and effectiveness can be assured by adherence to FDA’s “general controls” for medical devices. This includes compliance with the applicable portions of FDA’s Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. Some Class I devices require premarket clearance by FDA through the 510(k) premarket notification process described below but most are exempt from 510(k) premarket notification requirements.
Class II devices are moderate risk devices that present a higher risk than Class I devices. Class II devices subject to FDA’s general controls, and any other “special controls” deemed necessary by FDA to ensure the safety and effectiveness of the device, such as performance standards, product-specific guidance documents, special labeling requirements, patient registries or post-market surveillance. Premarket review and clearance by FDA for Class II devices is accomplished through the 510(k) premarket notification procedure, though certain Class II devices are exempt from this premarket review process. When a 510(k) is required, the manufacturer must submit to FDA a premarket notification submission demonstrating that the device is “substantially equivalent” to a legally marketed device, which in some cases may require submission of clinical data. If FDA determines that the device, or its intended use, is not substantially equivalent to a legally marketed device, then FDA will place the device, or the particular use of the device, into Class III, and the device sponsor must then fulfill much more rigorous premarketing requirements. Additionally, unless a specific exemption applies, 510(k) premarket notification submissions are subject to user fees.
Class III includes those with the greatest risk as they sustain or support life, are implanted, or present a potential unreasonable risk of illness or injury. In other words, Class III devices consist of devices deemed by FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device. The safety and effectiveness of Class III devices cannot be assured solely by general or special controls. Submission and FDA approval of a premarket approval, or PMA, application is generally required before marketing of a Class III device can proceed. The PMA process is much more demanding than the 510(k) premarket notification process. A PMA application, which is intended to demonstrate that the device is safe and effective, must be supported by extensive data, typically including data from preclinical studies and human clinical trials. Additionally, as with 510(k) submissions, unless subject to an exemption, PMA submissions are subject to user fees.
To determine the classification a product may be subject to you can use three different methods – searching for an appropriate product classification via FDA’s Product classification database; searching for a similar device by clearance or approval via FDA’s 510(k) Clearance Database, PMA Database, or De Novo Database; or searching for a similar device by device listing via FDA’s Establishment Registration and Device listing Database.
There are also De Novo and unclassified device types. Unclassified device types are pre-amendments devices (i.e., marketed prior to the Medical Device Amendments of 1976 but were not classified by the original classification panels) for which a classification regulation has not been promulgated. Until the unclassified device type has been formally classified and a regulation established by FDA, submission of a 510(k) premarket notification is generally required.
| 50 |
| Table of Contents |
De Novo classification, described in more detail below, provides a marketing pathway to classify novel medical devices for which general and/or special controls provide reasonable assurance of safety and effectiveness for the intended use but for which there is no legally marketed device upon which to base a determination of substantial equivalence predicate device (i.e., no predicate product, new intended use, or different technological characteristics that raise different questions of safety and effectiveness). Devices classified into Class I or Class II through a De Novo request may be marketed and used as predicates for other future submissions, where applicable.
510(k) Clearance
To obtain 510(k) clearance for a medical device, an applicant must submit to FDA a premarket notification submission demonstrating that the proposed device is “substantially equivalent” (i.e., as safe and effective) to a legally marketed device, known as a “predicate device.” A legally marketed predicate device may include a device that was legally marketed prior to May 28, 1976 for which a PMA is not required (known as a “pre-amendments device” based on the date of enactment of the Medical Device Amendments of 1976), a device that has been reclassified from Class III to Class II or Class I, a device that was found substantially equivalent through the 510(k) process or a device that was granted marketing authorization via the De Novo classification process that is not exempt from premarket notification requirement. A device is substantially equivalent if, with respect to the predicate device, it has the same intended use and has either (i) the same technological characteristics, or (ii) different technological characteristics, but the information provided in the 510(k) submission demonstrates that the device does not raise new questions of safety and effectiveness and is at least as safe and effective as the legally marketed predicate device. A showing of substantial equivalence sometimes, but not always, can require clinical data and non-clinical bench performance data, including engineering performance testing, sterility, electromagnetic compatibility, software validation, biocompatibility evaluation, among other data.
Before FDA will accept a 510(k) submission for substantive review, FDA will first assess whether the submission satisfies a minimum threshold of acceptability. If FDA determines that the 510(k) submission is incomplete, then FDA will issue a “Refuse to Accept” letter which generally outlines the information FDA believes is necessary to permit a substantive review and to reach a determination regarding substantial equivalence. An applicant must submit the requested information before FDA will proceed with additional review of the submission. Once the 510(k) submission is accepted for review, by regulation, FDA has 90 days to review and issue a determination. As a practical matter, clearance often takes longer. FDA may require additional information, including additional clinical and non‑clinical data, to make a determination regarding substantial equivalence.
If FDA agrees that the device is substantially equivalent to a predicate device currently on the market, then it will grant 510(k) clearance to commercially market the device. If FDA determines that the device is “not substantially equivalent” to a previously cleared device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the De Novo process.
After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, PMA approval. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance. Many minor modifications today are accomplished by a “letter to file” in which the manufacture documents the rationale for the change and why a new 510(k) is not required. However, FDA may review such letters to file to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
PMA Approval
A PMA must be submitted to FDA for any device that is classified in Class III or otherwise cannot be cleared through the 510(k) process (although FDA has discretion to continue to allow certain pre-amendment Class III devices to use the 510(k) process). PMA applications must be supported by, among other things, valid scientific evidence demonstrating the safety and effectiveness of the device, which typically requires extensive data, including technical, preclinical, clinical and manufacturing data (e.g., study protocols, adverse reactions and complications, device failures and replacements, patient information, patient complaints, tabulations of data from all individual subjects, results of statistical analyses), and non-clinical laboratory or safety studies (e.g., microbiology, toxicology, immunology, biocompatibility, stress, wear, shelf life, and other laboratory or animal tests). The PMA must also contain a full description of the device and its components, a full description of the methods, facilities, and controls used for manufacturing, and proposed labeling.
| 51 |
| Table of Contents |
Following receipt of a PMA application, once FDA determines that the application is sufficiently complete to permit a substantive review, FDA will formally accept the application for review. FDA, by statute and by regulation, has 180-days to review an “accepted” PMA application, although the review of an application more often occurs over a significantly longer period of time, and can take up to several years. During the review period, FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside FDA may be convened to review and evaluate the application and provide recommendations to FDA as to the approvability of the device. FDA may or may not accept the panel’s recommendation. In addition, FDA will generally conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the QSR.
If FDA evaluations of both the PMA application and the manufacturing facilities are favorable, FDA will either issue an approval letter or an approvable letter, which usually contains a number of conditions that must be met in order to secure final approval of the PMA. If FDA’s evaluation of the PMA or manufacturing facilities is not favorable, FDA will deny approval of the PMA or issue a not approvable letter. A not approvable letter will outline the deficiencies in the application and, where practical, will identify what is necessary to make the PMA approvable. FDA may also determine that additional clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted. Once granted, PMA approval may be withdrawn by FDA if compliance with post-approval requirements, conditions of approval or other regulatory standards is not maintained or problems are identified following initial marketing.
In approving a PMA, FDA may also require some form of post-market surveillance when necessary to protect the public health or to provide additional safety and effectiveness data for the device. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and makes periodic reports to FDA on the clinical status of those patients.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of a PMA-approved device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel.
De Novo Classification
Medical device types that FDA has not previously classified as Class I, II or III are automatically classified into Class III regardless of the level of risk they pose. The Food and Drug Administration Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, called the “Request for Evaluation of Automatic Class III Designation,” or the de novo classification procedure. This procedure allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, FDA may reject the reclassification petition if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed. Under the Medical Device User Fee Amendments of 2017 (MDUFA IV), FDA’s goal is to make a decision about a De Novo request in 150 review days. Review days are calculated as the number of calendar days between the date the De Novo request was received by FDA and the date of FDA’s decision, excluding the days a request was on hold for an Additional Information request.
| 52 |
| Table of Contents |
It is our current belief that our initial product, the KnowU and UBand glucose monitoring products, are appropriate for a de novo classification request.
Breakthrough Devices Program
The Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency’s mission to protect and promote public health.
The Breakthrough Devices Program replaces the Expedited Access Pathway and Priority Review for medical devices and offers manufacturers an opportunity to interact with FDA to efficiently address topics as they arise during the premarket review phase. FDA considers devices granted designation under the Expedited Access Pathway to be part of the Breakthrough Devices Program.
All requests for Breakthrough designation must be submitted prior to submitting a marketing submission and can be revoke by FDA at any time. Additionally, devices eligible for Breakthrough Device designation must (1) provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions; and (2) meet at least one of the following: (i) represent breakthrough technology; (ii) have no approved or cleared alternatives that exist; (iii) offer significant advantages over existing approved or cleared alternatives; or (iv) whose availability is in the best interest of patients.
We may pursue the Breakthrough Devices Program for the KnowU and UBand glucose monitoring products.
Clinical Studies
When FDA clearance or approval of a Class I, Class II or Class III device requires human clinical trials, and if the device presents a “significant risk” to human health, then the device sponsor is required to file an IDE application with FDA and obtain IDE approval prior to commencing the human clinical trial. If the device is considered a “non-significant risk,” IDE submission to FDA is not required but must still follow IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and complying with labeling and record keeping requirements. Instead, only approval from the Institutional Review Board, or IRB, overseeing the investigation at each clinical trial site is required. Human clinical studies are generally required in connection with approval of Class III devices and may be required for Class I and II devices.
Regardless of the degree of risk presented by the medical device, clinical studies must be approved by, and conducted under the oversight of, an IRB for each clinical site. The IRB is responsible for the initial and continuing review of the IDE and may impose additional requirements for the conduct of the study. If an IDE application is approved by FDA and one or more IRBs, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by FDA.
The sponsor is also required to comply with the applicable FDA requirements during the clinical trial (e.g., trial monitoring, selecting clinical investigators and providing them with the investigational plan, ensuring IRB review, adverse event reporting, record keeping and prohibitions on the promotion of investigational devices, or on making safety or effectiveness claims for them). The sponsor may transfer some or all of its obligations related to a clinical study to a third-party but is ultimately responsible for compliance regardless of whether these obligations are contractually transferred.
The clinical investigators in the clinical study are also subject to FDA’s regulations and must obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of the investigational device, and comply with all reporting and recordkeeping requirements. Additionally, after a trial begins, we, FDA, or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.
| 53 |
| Table of Contents |
FDA or the IRB at each institution at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product in the United States.
Post-Marketing Restrictions and Enforcement
After a device is placed on the market, numerous regulatory requirements apply. These include, but are not limited to:
|
| • | Product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
|
| • | Reporting of potential device shortages in some circumstances, including during a public health emergency; |
|
| • | Compliance with QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; |
|
| • | unannounced routine or for-cause device facility inspections by FDA, which may include our suppliers’ facilities; |
|
| • | labeling regulations and FDA prohibitions against the promotion of products for uncleared or unapproved “off-label” uses; |
|
| • | clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; |
|
| • | corrections and removal reporting regulations, which require that manufacturers report to FDA field corrections or removals if undertaken to reduce a risk to health posed by a device or to remedy a violation of the FFDCA that may present a risk to health; |
|
| • | medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
|
| • | post-approval restrictions or conditions, including post-approval study commitments; |
|
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
|
| • | FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and |
|
| • | regulations pertaining to voluntary recalls. |
Advertising and promotion of medical devices, in addition to being regulated by FDA, are also regulated by the Federal Trade Commission (the “FTC”) as well as comparable state consumer protection laws. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. If FDA determines that our promotional materials or training constitutes promotion of an unapproved or uncleared use, then it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved or uncleared use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged, and adoption of the products would be impaired.
| 54 |
| Table of Contents |
In addition, under FDA medical device reporting (“MDR”) regulations, medical device manufacturers are required to report to FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or a similar device of such manufacturer were to recur. The decision to file an MDR involves a judgment by the manufacturer. If FDA disagrees with the manufacturer’s determination, FDA can take enforcement action.
The MDR requirements also extend to health care facilities that use medical devices in providing care to patients, or “device user facilities,” which include hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, or outpatient treatment facilities, but not physician offices. A device user facility must report any device-related death to both FDA and the device manufacturer, or any device-related serious injury to the manufacturer (or, if the manufacturer is unknown, to FDA) within 10 days of the event. Device user facilities are not required to report device malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur but may voluntarily report such malfunctions through MedWatch, FDA’s Safety Information and Adverse Event Reporting Program.
FDA also has the authority to require the recall of commercialized medical device products in the event of material deficiencies or defects in design or manufacture. The authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious adverse health consequences or death. Manufacturers may, under their own initiative, recall a product if any distributed devices fail to meet established specifications, are otherwise misbranded or adulterated under the FFDCA, or if any other material deficiency is found. FDA requires that certain classifications of recalls be reported to FDA within ten working days after the recall is initiated.
The failure to comply with applicable regulatory requirements can result in enforcement action by FDA, which may include any of the following sanctions:
|
| · | warning letters, fines, injunctions or civil penalties; |
|
|
|
|
|
| · | recalls, detentions or seizures of products; |
|
|
|
|
|
| · | operating restrictions; |
|
|
|
|
|
| · | delays in the introduction of products into the market; |
|
|
|
|
|
| · | total or partial suspension of production; |
|
|
|
|
|
| · | delay or refusal of the FDA or other regulators to grant 510(k) clearance, PMA approvals, or other marketing authorization to new products; |
|
|
|
|
|
| · | withdrawals of marketing authorizations; or |
|
|
|
|
|
| · | in the most serious cases, criminal prosecution. |
To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled and unannounced inspections by FDA, and these inspections may include the manufacturing facilities of subcontractors.
Federal Trade Commission Regulatory Oversight
Our advertising for our products and services is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission (the “FTC”) as well as comparable state consumer protection laws. Under the Federal Trade Commission Act (the “FTC Act”), the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
| 55 |
| Table of Contents |
International
Our international sales are subject to regulatory requirements in the countries in which our products are sold. The regulatory review process varies from country to country and may in some cases require the submission of clinical data.
DIRECTORS AND EXECUTIVE OFFICERS
The following sets forth, as of September 1, 2023, the name, age, position and certain information of each executive officer and director and the tenure in office of each director of the Company.
Identification of Directors and Executive Officers
The following table sets forth certain information about our current directors and executive officers:
| Name |
| Age |
| Director/Executive Officer |
|
| Ronald P. Erickson |
| 79 |
| Chairman and Chief Executive Officer |
|
| Peter Conley |
| 68 |
| Chief Financial Officer and SVP Intellectual Property |
|
| Jon Pepper |
| 72 |
| Director |
|
| Ichiro Takesako |
| 64 |
| Director |
|
| William A. Owens |
| 83 |
| Director |
|
Set forth below is information regarding our directors and executive officers.
Ronald P. Erickson. Mr. Erickson was appointed as Chief Executive Officer in January 2023. Mr. Erickson previously served as our Chief Executive Officer from November 2009 to April 2018. He has served as Chairman of the Board from 2004 to 2011 and from 2015 to the present. A senior executive with more than 30 years of experience in the technology, telecommunications, software, and digital media industries, Mr. Erickson was the founder of our company. He is formerly Chairman, CEO and Co-Founder of Blue Frog Media, a mobile media and entertainment company; Chairman and CEO of eCharge Corporation, an Internet-based transaction procession company; Chairman, CEO and Co-founder of GlobalTel Resources, a provider of telecommunications services; Chairman, Interim President and CEO of Egghead Software, Inc., a software reseller where he was an original investor; Chairman and CEO of NBI, Inc.; and Co-founder of MicroRim, Inc., the database software developer. Earlier, Mr. Erickson practiced law in Seattle and worked in public policy in Washington, DC and New York, NY. Additionally, Mr. Erickson has been an angel investor and board member of a number of public and private technology companies. In addition to his business activities, Mr. Erickson was Chairman and a member of the Board of Trustees of Central Washington University where he received his BA degree from 2010 to 2021. He also holds a MA from the University of Wyoming and a JD from the University of California, Davis. He is licensed to practice law in the State of Washington. Mr. Erickson is our founder and was appointed as a director because of his extensive experience in developing technology companies.
Peter J. Conley. Mr. Conley has served as our Chief Financial Officer and SVP Intellectual Property since May 2022. In addition, Mr. Conley currently serves as Senior Managing Director and Head of Intellectual Property Banking at Boustead Securities, LLC, a position he has held since October 2014, where he provides equity financing and M&A advisory services to small-cap public companies. Prior to that, from 2012 to 2016, Mr. Conley was a cofounder and Chief Operating Officer of ipCreate, a global IP development and innovation services company serving large multinational companies. He also served as managing director of ipCapital Venture Group, where he provided IP strategy and venture advisory services. During his career spanning more than 35 years, Mr. Conley has held leadership roles at MDB Capital Group, The Analytiq Group / RDEX Research, Roth Capital Partners, and Lehman Brothers. He was on the founding team and Head of Equity Capital Markets at E*Offering, the investment bank of E*Trade. Mr. Conley attended the University of Hawaii at Manoa and the University of London, Center for Financial & Management Studies, SOAS.
| 56 |
| Table of Contents |
Jon Pepper. Mr. Pepper has served as an independent director since April 2006. Mr. Pepper founded Pepcom, a company that become the industry leader at producing press-only technology showcase events around the country and internationally, in 1980. He sold his stake in the corporation and retired as a partner at the end of 2018. Prior to that, Mr. Pepper started the DigitalFocus newsletter, a ground-breaking newsletter on digital imaging that was distributed to leading influencers worldwide. Mr. Pepper has been closely involved with the high technology revolution since the beginning of the personal computer era. He was formerly a well-regarded journalist and columnist. His work on technology subjects appeared in The New York Times, Fortune, PC Magazine, Men’s Journal, Working Woman, PC Week, Popular Science and many other well-known publications. Mr. Pepper was educated at Union College in Schenectady, New York and the Royal Academy of Fine Arts in Copenhagen. He continues to be active in non-profit work and private company boards and in 2017 founded Mulberry Tree Films, a non-profit that supports independent high-quality documentary films and other publishing and creative projects that are oriented toward increasing the understanding of human potential and creativity. Mulberry Tree funded and produced the acclaimed documentary, “The Gates of Shinto” and is currently at work on additional projects. Mr. Pepper was appointed as a director because of his marketing skills with technology companies. Mr. Pepper was appointed as a director because of his marketing skills with technology companies.
Ichiro Takesako. Mr. Takesako has served as a director since December 2012. Mr. Takesako has held executive positions with Sumitomo Precision Products Co., Ltd, or Sumitomo, and its affiliates since 1983. In the past few years, Mr. Takesako has held the following executive position in Sumitomo and its affiliates: in June 2008, he was appointed as General Manager of Sales and Marketing Department of Micro Technology Division; in April 2009, he was appointed as General Manager of Overseas Business Department of Micro Technology Division, in charge of M&A activity of certain business segment and assets of Aviza Technology, Inc.; in July 2010, he was appointed as Executive Director of SPP Process Technology Systems, a 100% owned subsidiary of Sumitomo Precision Products at the time; in August 2011, he was appointed as General Manager, Corporate Strategic Planning Group; in January 2013, he was appointed as Chief Executive Officer of M2M Technologies, Inc., a company invested by Sumitomo Precision products; in April 2013, has was appointed as General Manager of Business Development Department, in parallel of CEO of M2M Technologies, Inc.; in April 2014, he was relieved from General Manager of Business Development Department and is responsible for M2M Technologies Inc. as its CEO; in March 2017, he established At Signal, Inc. which took over the entire business operation from M2M Technologies, Inc.; and in April 2017, he was appointed as Chief Executive Officer of At Signal Inc. Mr. Takesako graduated from Waseda University, Tokyo, Japan where he majored in Social Science and graduated with a Degree of Bachelor of Social Science. Mr. Takesako was appointed as a director based on his previous position with Sumitomo and Sumitomo’s previous significant partnership with our company. Mr. Takesako was appointed as a director based on his previous position with Sumitomo and Sumitomo’s previous significant partnership with our company.
William A. Owens. William A. Owens is the co-founder and executive chairman of Red Bison Technology Group, a company which installs and operates high speed telecoms networks and technology in large office buildings. He is the Chairman of Visionary Vehicles which is building a series of automobiles focused on electric and hydrogen powered cars, Kyrrex which is a successful and growing Crypto Currency Exchange operating in Europe, and Massif, an electric bicycle company. Owens serves on the board of directors of the Public Companies, Siply, Know Labs, and Compass, and is a director of the private companies: TruU, Tethr, ViruSight, Prism, Steel Grove, JennyCo, Axxess Capital, Versium, and Viome. Owens was the chairman of the board of CenturyLink Telecom (now Lumen), the third largest telecommunications company in the United States and SAP USA. Owens is on the board of trustees of Seattle University, and the Fiscal Responsibility Amendment (CFFRA) Association which aims to establish a balanced budget amendment to the US Constitution. He is a member of the Council of Foreign Relations. He is the Founder and senior General on a China US forum to bring 4 star generals together for China US cooperation. He is a Senior Fellow at Stimson Institute.
From 2007 to 2015, Owens was the Chairman and Senior Partner of AEA Investors Asia, a private equity firm located in Hong Kong, and Vice Chairman of the NYSE for Asia. Owens also served as the Chairman of Eastern Airlines. He has served on over 25 public boards including Daimler, British American Tobacco, Telstra, Nortel Networks, and Polycom.
| 57 |
| Table of Contents |
Owens was the CEO of Nortel, a fortune 500 company, the CEO/Chairman of Teledesic, a Bill Gates/Craig McCaw company bringing worldwide broadband through an extensive satellite network and was the President of Science Applications International Corporation (SAIC). He also served on the boards of the not-for-profit organizations; Fred Hutchinson Cancer Research Center, Carnegie Corporation of New York, Brookings Institution, East West Institute, and RAND Corporation.
Owens is a retired four-star US Navy Admiral. He was Vice Chairman of the Joint Chiefs of Staff, the second-ranking United States military officer in the US, with responsibility for reorganizing and restructuring the armed forces in the post- Cold War era. He is widely recognized for bringing commercial high-grade technology into the Department of Defense for military applications. Owens was the architect of the Revolution in Military Affairs (RMA), an advanced systems technology approach to military operations, the most significant change in the system of requirements, budgets and technology for the four armed forces since World War II. Owens was Commander of the U.S. Sixth Fleet from 1990 to 1992, which included Operation Desert Storm. Owens also served as the deputy Chief of Naval Operations for Resources and Requirements. Owens was the Senior Military Assistant to two Secretaries of Defense (Cheney and Carlucci) and served in the Office of Program Appraisal for the Secretary of the Navy. He began his military career as a nuclear submariner. He served on four strategic nuclear-powered submarines and three nuclear attack submarines, including tours as Commanding Officer of the USS Sam Houston, USS Michigan, and USS City of Corpus Christi.
Owens is a 1962 honor graduate of the United States Naval Academy in mathematics, holds bachelors and master’s degrees in politics, philosophy and economics from Oxford University, and a masters degree in management from George Washington University. He has written more than 50 articles on national security and authored the book “High Seas.”. His book, “Lifting the Fog of War,” was published in April 2000 with a revision published in Mandarin in 2009. And his book “China-US 2039: The Endgame?” was published in 2019 in both English and Mandarin.
Owens has received numerous recognitions and awards: the “Légion d’Honneur” by France, and the highest awards given to foreigners by the countries of Indonesia and Sweden. He was named as one of The 50 Most Powerful People in Networking by Network World, one of the 100 Best Board Members in the United States for 2011 and again in 2016 awarded by NACD, and the Intrepid Salute Award in recognition of his business achievements and support of important philanthropic activities. Owens is active in philanthropy to foster Chinese – American relations including dialogues between the most senior retired officers in the United States and Chinese militaries. He is a North Dakota’s Roughriders recipients, the award given annually to the most prominent North Dakotans. Admiral Owens was appointed as a director of Know Labs because of his financials and governance skills.
Term of Office
Our directors currently have terms which will end at our next annual meeting of stockholders or until their successors are elected and qualify, subject to their prior death, resignation or removal. Officers serve at the discretion of the Board.
Family Relationship
There are no family relationships among any of our officers or directors.
| 58 |
| Table of Contents |
Involvement in Certain Legal Proceedings
To the best of our knowledge, except as described below, none of our directors or executive officers has, during the past ten years:
|
| · | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
|
|
|
|
|
| · | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
|
|
|
|
|
| · | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
|
|
|
|
|
| · | been found by a court of competent jurisdiction in a civil action or by the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
|
|
|
|
|
| · | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
|
|
|
|
|
| · | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
| 59 |
| Table of Contents |
Our Board’s Role in Risk Oversight
Our Board oversees that the assets of our company are properly safeguarded, that the appropriate financial and other controls are maintained, and that our business is conducted wisely and in compliance with applicable laws and regulations and proper governance. Included in these responsibilities is the Board’s oversight of the various risks facing our company. In this regard, our Board seeks to understand and oversee critical business risks. Our Board does not view risk in isolation. Risks are considered in virtually every business decision and as part of our business strategy. Our Board recognizes that it is neither possible nor prudent to eliminate all risk. Indeed, purposeful and appropriate risk-taking is essential for our company to be competitive on a global basis and to achieve our objectives.
While the Board oversees risk management, company management is charged with managing risk. Management communicates routinely with the Board and individual directors on the significant risks identified and how they are being managed. Directors are free to, and indeed often do, communicate directly with senior management.
Our Board administers its risk oversight function as a whole by making risk oversight a matter of collective consideration; however, much of the work is delegated to committees, which will meet regularly and report back to the full Board. The audit committee oversees risks related to our financial statements, the financial reporting process, accounting and legal matters, the compensation committee evaluates the risks and rewards associated with our compensation philosophy and programs, and the nominating and corporate governance committee evaluates risks associated with management decisions and strategic direction.
Attendance at Annual Meetings of Stockholders
We expect that all of our Board members attend our annual meetings of stockholders in the absence of a showing of good cause for failure to do so. Ichiro Takesako and William A. Owens attended our 2022 annual meeting of stockholders in person or by telephone.
Board Meetings and Committees
During our last fiscal year, each of our directors attended at least 75% of the aggregate of (i) the total number of Board meetings and (ii) the total number of meetings of the committees on which the director served.
Independent Directors
NYSE American’s rules generally require that a majority of an issuer’s board of directors must consist of independent directors. Our Board currently consists of four (4) directors, three (3) of whom, Messrs. Owens, Pepper and Takesako, are independent within the meaning of NYSE American rules.
Committees of the Board of Directors
Our Board has established an audit committee, a compensation committee and a nominating and corporate governance committee, each with its own charter approved by the Board. Each committee’s charter is available on our website at www.knowlabs.co. In addition, our Board may, from time to time, designate one or more additional committees, which shall have the duties and powers granted to it by our Board.
Audit Committee
William A. Owens, Jon Pepper and Ichiro Takesako, each of whom satisfies the “independence” requirements of Rule 10A-3 under the Exchange Act and NYSE American’s rules, serve on our audit committee, with Mr. Pepper serving as the chairman. Our Board has determined that Mr. Owens qualifies as an “audit committee financial expert” as defined by applicable SEC rules. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of the Company.
The audit committee is responsible for, among other things: (i) retaining and overseeing our independent accountants; (ii) assisting the Board in its oversight of the integrity of our financial statements, the qualifications, independence and performance of our independent auditors and our compliance with legal and regulatory requirements; (iii) reviewing and approving the plan and scope of the internal and external audit; (iv) pre-approving any audit and non-audit services provided by our independent auditors; (v) approving the fees to be paid to our independent auditors; (vi) reviewing with our chief executive officer and principal financial officer and independent auditors the adequacy and effectiveness of our internal controls; (vii) reviewing hedging transactions; and (viii) reviewing and assessing annually the audit committee’s performance and the adequacy of its charter. The audit committee is also responsible for preparing a report to be included with this Proxy Statement. Our audit committee met 4 times during the last fiscal year.
| 60 |
| Table of Contents |
Compensation Committee
William A. Owens, Jon Pepper and Ichiro Takesako, each of whom satisfies the “independence” requirements of Rule 10C-1 under the Exchange Act and NYSE American’s rules, serve on our compensation committee, with Mr. Owens serving as the chairman. The members of the compensation committee are also “non-employee directors” within the meaning of Section 16 of the Exchange Act. The compensation committee assists the Board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers.
The compensation committee is responsible for, among other things: (i) reviewing and approving the remuneration of our executive officers; (ii) making recommendations to the Board regarding the compensation of our independent directors; (iii) making recommendations to the Board regarding equity-based and incentive compensation plans, policies and programs; and (iv) reviewing and assessing annually the compensation committee’s performance and the adequacy of its charter. Our compensation committee met 4 times during the last fiscal year.
No member of our compensation committee is or has been our current or former officer or employee. None of our executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served as a director or member of our compensation committee during the fiscal year ended September 30, 2022.
Nominating and Corporate Governance Committee
William A. Owens, Jon Pepper and Ichiro Takesako, each of whom satisfies the “independence” requirements of NYSE American’s rules, serve on our nominating and corporate governance committee, with Mr. Pepper serving as the chairman. The nominating and corporate governance committee assists the Board in selecting individuals qualified to become our directors and in determining the composition of the Board and its committees.
The nominating and corporate governance committee is responsible for, among other things: (i) identifying and evaluating individuals qualified to become members of the Board by reviewing nominees for election to the Board submitted by stockholders and recommending to the Board director nominees for each annual meeting of stockholders and for election to fill any vacancies on the Board; (ii) advising the Board with respect to Board organization, desired qualifications of Board members, the membership, function, operation, structure and composition of committees (including any committee authority to delegate to subcommittees), and self-evaluation and policies; (iii) advising on matters relating to corporate governance and monitoring developments in the law and practice of corporate governance; (iv) overseeing compliance with our code of ethics; and (v) approving any related party transactions.
The nominating and corporate governance committee’s methods for identifying candidates for election to our Board (other than those proposed by our stockholders, as discussed below) include the solicitation of ideas for possible candidates from a number of sources – members of our Board, our executives, individuals personally known to the members of our Board, and other research. The nominating and corporate governance committee may also, from time-to-time, retain one or more third-party search firms to identify suitable candidates.
| 61 |
| Table of Contents |
In making director recommendations, the nominating and corporate governance committee may consider some or all of the following factors: (i) the candidate’s judgment, skill, and experience with other organizations of comparable purpose, complexity and size, and subject to similar legal restrictions and oversight; (ii) the interplay of the candidate’s experience with the experience of other Board members; (iii) the extent to which the candidate would be a desirable addition to the Board and any committee thereof; (iv) whether or not the person has any relationships that might impair his or her independence; and (v) the candidate’s ability to contribute to the effective management of the Company, taking into account the needs of the Company and such factors as the individual’s experience, perspective, skills and knowledge of the industry in which we operate.
A stockholder may nominate one or more persons for election as a director at an annual meeting of stockholders if the stockholder complies with the notice and information provisions contained in our Bylaws. Such notice must be received in writing to our Company not later than the close of business fourteen (14) days nor earlier than the close of business eighty (80) days prior to the first anniversary of the preceding year’s annual meeting; provided, however, that if less than twenty-one (21) days’ notice of the meeting is given to stockholders, such writing shall be received by the Secretary of the Corporation not later than the close of the seventh (7th) day following the day on which notice of the meeting was mailed to stockholders. In addition, stockholders furnishing such notice must be a holder of record on both (i) the date of delivering such notice and (ii) the record date for the determination of stockholders entitled to vote at such meeting.
Code of Ethics
We have adopted a code of ethics that applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer. Such code of ethics addresses, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, and reporting of violations of the code.
A copy of the code of ethics has been filed as an exhibit to our registration statement on Form S-1, as amended (File No. 333-266423), initially filed with the SEC on July 29, 2022, and is also available on our website at www.knowlabs.io. We are required to disclose any amendment to, or waiver from, a provision of our code of ethics applicable to our principal executive officer, principal financial officer, principal accounting officer, controller, or persons performing similar functions. We intend to use our website as a method of disseminating this disclosure as well as by SEC filings, as permitted or required by applicable SEC rules. Any such disclosure will be posted to our website within four (4) business days following the date of any such amendment to, or waiver from, a provision of our code of ethics.
Communication with our Board of Directors
Our stockholders and other interested parties may communicate with our Board of Directors by sending written communication in an envelope addressed to “Board of Directors” in care of the Secretary, 500 Union Street, Suite 810, Seattle, Washington 98101.
Section 16(a) Beneficial Ownership Reporting Compliance
Our executive officers, directors and 10% stockholders are required under Section 16(a) of the Exchange Act to file reports of ownership and changes in ownership with the SEC. Copies of these reports must also be furnished to us.
Based solely on a review of copies of reports furnished to us, as of September 30, 2022 our executive officers, directors and 10% holders complied with all filing requirements except as follows:
Jon Pepper filed a Form 4 on January 10, 2022 that was required to be filed on January 7, 2022.
Ichiro Takesako-
Filed a Form 4 on January 10, 2022 that was required to be filed on January 7, 2022.
Filed a Form 4 on March 2, 2022 that was required to be filed on February 24, 2022.
Filed a Form 4 on June 7, 2022 that was required to be filed on May 30, 2022.
William A. Owens-
Filed a Form 4 on January 10, 2022 that was required to be filed on January 7, 2022.
Filed a Form 4 on February 4, 2022 that was required to be filed on January 27, 2022.
| 62 |
| Table of Contents |
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named persons (our “named executive officers”) for services rendered in all capacities during the years ended September 30, 2022 and September 30, 2021, respectively. The Company meets the requirements of a “smaller reporting company” and has utilized the scaled reporting requirements available to qualifying companies. No other executive officers received total annual salary and bonus compensation in excess of $100,000.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| All |
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
| Stock |
|
| Option |
|
| Other |
|
|
|
|
||||||
|
|
|
|
|
|
| Salary |
|
| Bonus |
|
| Awards |
|
| Awards |
|
| Compensation |
|
| Total |
|
||||||
| Name |
| Principal Position |
|
| ($) |
|
| ($) |
|
| ($) |
|
| ($) (4) |
|
| ($) |
|
| ($) |
|
|||||||
| Ronald P. Erickson (1) |
| Chief Executive Officer and Chairman of the Board |
| Fiscal year 2022 |
| $ | 474,475 |
|
| $ | - |
|
| $ | - |
|
| $ | 1,748,231 |
|
| $ | - |
|
| $ | 2,222,706 |
|
|
|
|
|
| Fiscal year 2021 |
| $ | 366,042 |
|
| $ | - |
|
| $ | - |
|
| $ | 1,811,691 |
|
| $ | - |
|
| $ | 2,177,733 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Phillip A. Bosua (2) |
| Former Chief Executive Officer |
| Fiscal year 2022 |
| $ | 1,437,926 |
|
| $ | - |
|
| $ | - |
|
| $ | 865,601 |
|
| $ | 91,500 |
|
| $ | 2,395,027 |
|
|
|
|
|
| Fiscal year 2021 |
| $ | 413,760 |
|
| $ | 250,000 |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | 663,760 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Peter J. Conley (3) |
| Chief Financial Officer and SVP Intellectual Property |
| Fiscal year 2022 |
| $ | 110,000 |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | 110,000 |
|
|
|
|
|
| Fiscal year 2021 |
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
(1) During the years ended September 30, 2022 and 2021, the Compensation Committee and the Board compensated Ronald P. Erickson with an annual salary of $215,000 from October 1, 2020 to March 31, 2021. From April 1, 2021 to March 15, 2022, the annual compensation was $300,000 and to $325,000 from March 15, 2022 to September 30, 2022. The Compensation Committee and the Board of Particle, Inc. compensated Ronald P. Erickson with a salary of $105,000 for the year ended September 30, 2021. From December 14, 2022, Mr. Erickson has been compensated with an annual salary of $375,000. See “Outstanding Equity Awards at Year-End” for a discussion of option award compensation.
(2) Mr. Bosua resigned effective January 23, 2023. In connection with Mr. Bosua’s resignation on January 23, 2023, the Company and Mr. Bosua entered into a Separation and Release Agreement. During the years ended September 30, 2022 and 2021, the Compensation Committee and the Board compensated Phillip A. Bosua at an annual salary of $260,000 from October 1, 2020 to March 31, 2021. From April 1, 2021 to September 30, 2022, the annual compensation was $350,000. Mr. Bosua was paid $1,097,928 in compensation for services provided to AI Mind, a wholly owned subsidiary of the Company, in connection with the development of NFT sales for the year ended September 30, 2022. The Compensation Committee and the Board of Particle, Inc. compensated Phillip A. Bosua with a salary of $105,000 for the year ended September 30, 2021. Mr. Bosua received $91,500 in amounts paid or reimbursed for rent expenses in connection with the development of NFT sales for the Company’s wholly owned subsidiary, AI Mind, for the year ended September 30, 2022. See the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Digital Asset Sales” and Note 4 to the Notes to our Consolidated Financial Statements, each included in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, for more information about NFT sales.
Mr. Bosua resigned from the Board of Directors and from his position as Chief Executive Officer on January 23, 2023. Mr. Bosua is party to a Separation and Release Agreement with the Company, pursuant to which he was entitled to receive severance payments. Such payments are described in greater detail below under “Employment and Separation Agreements” and such amounts will be disclosed in the summary compensation table for the fiscal year ended September 30, 2023.
During the year ended September 30, 2021, we paid a $250,000 bonus for Mr. Bosua. See “Outstanding Equity Awards at Year-End” for a discussion of option award compensation.
(3) Mr. Peter J. Conley has served as our Chief Financial Officer and SVP Intellectual Property since May 2022. During the year ended September 30, 2022, the Compensation Committee and the Board compensated Mr. Conley with an annual salary of $300,000 from May 20, 2022 to September 30, 2022. From December 14, 2022, Mr. Conley has been compensated with an annual salary of $325,000.
(4) These amounts reflect the aggregate grant date fair value of awards granted in the fiscal year ended September 30, 2022, as required by Regulation S-K Item 402(n)(2), computed in accordance with the FASB Accounting Standards Codification Topic 718 (“FASB ASC Topic 718”). All assumptions made in the valuations are contained and described in footnote 10 to the Company’s financial statements for Fiscal 2022 contained in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, filed with the SEC on December 20, 2022. The amounts shown in the table reflect the total fair value on the date of grant and do not necessarily reflect the actual value, if any, that may be realized by the listed executives.
| 63 |
| Table of Contents |
Employment and Separation Agreements
On April 10, 2018, we entered into an amended employment agreement for Ronald P. Erickson which amends our employment agreement with him dated July 1, 2017. The employment agreement provides for a base salary of $180,000 per year, which was increased to $215,000 from May 1, 2020 to March 31, 2021, to $300,000 from April 1, 2021 to March 15, 2022 and to $325,000 from March 15, 2022 to September 30, 2022. The compensation committee and the board of Particle, Inc., our wholly-owned subsidiary, compensated Mr. Erickson with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. Mr. Erickson will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. The employment agreement is for an initial term of 12 months (subject to earlier termination) and will be automatically extended for additional 12-month terms unless either party notifies the other party of its intention to terminate the employment agreement at least ninety (90) days prior to the end of the initial term or renewal term. If our company terminates Mr. Erickson’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Erickson terminates his employment at any time for “good reason” or due to a “disability,” Mr. Erickson will be entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months. On January 23, 2023, the Board appointed Mr. Erickson to the position of Chief Executive Officer of the Company. Mr. Erickson was appointed to serve until his successor is duly elected.
On April 10, 2018, we entered into an employment agreement with Phillip A. Bosua reflecting his appointment as Chief Executive Officer. The employment agreement provided for a base salary of $225,000 per year, which was increased to $260,000 from May 1, 2020 to March 31, 2021 and to $350,000 from April 1, 2021 to September 30, 2022. The compensation committee and the board of directors of Particle, Inc., our wholly-owned subsidiary, compensated Phillip A. Bosua with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. Mr. Bosua also received 500,000 shares of common stock valued at $0.33 per share and was entitled to bonuses and equity awards at the discretion of the Board or a committee of the Board. Mr. Bosua was entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. The employment agreement was for an initial term of 12 months (subject to earlier termination) and was automatically extended for additional 12-month terms unless either party notified the other party of its intention to terminate the employment agreement at least ninety (90) days prior to the end of the initial term or renewal term. If our company terminated Mr. Bosua’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Bosua terminated his employment at any time for “good reason” or due to a “disability,” Mr. Bosua was entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months.
On January 23, 2023, Mr. Bosua resigned from the Board and from his position as Chief Executive Officer of the Company. In connection with his resignation, we entered into a Separation and Release Agreement (the “Separation Agreement”) with Mr. Bosua containing customary terms and mutual releases, pursuant to which Mr. Bosua is entitled receive a $400,000 severance payment and benefits pursuant to his prior employment agreement. Pursuant to the Separation Agreement, Mr. Bosua’s outstanding stock options ceased vesting as of January 23, 2023, and all vested stock options remain exercisable through January 23, 2024. Mr. Bosua has been engaged as a consultant to the Company for a period of one year at a rate of $10,000 per month. Mr. Bosua also entered into a lock up and leak out agreement with respect to 3,005,000 common shares owned by Mr. Bosua and shares issuable upon exercise of his vested option awards. During the period commencing March 17, 2023 through March 17, 2024, Mr. Bosua may sell no more than 1,500,000 shares. During the period commencing April 1, 2024 through June 30, 2026, Mr. Bosua may sell no more than 375,000 shares per quarter (or 1,500,000 shares per year), unless the stock price of the Company’s common stock exceeds $5.00 per share on the NYSE American (the “Stock Price Threshold”), then Mr. Bosua may sell a maximum of 750,000 shares during any such quarter that the Stock Price Threshold is met. Notwithstanding the foregoing, any lock-up or leak-out restrictions are waived for any sales of shares from Mr. Bosua to Todd Baszucki.
| 64 |
| Table of Contents |
On May 13, 2022, we entered into an employment agreement with Peter J. Conley reflecting his appointment as our Chief Financial Officer and Senior Vice President, Intellectual Property. The employment agreement provides for a base salary of $300,000 and Mr. Conley may also be entitled to bonuses from time to time as determined by our Board or our compensation committee in their sole discretion. Mr. Conley is eligible to participate in all our employee benefit plans, policies and arrangements that are applicable to other executive officers, as such plans, policies and arrangements may exist or change from time to time at our discretion. We will reimburse Mr. Conley for reasonable travel, entertainment and other expenses he incurs in the furtherance of his duties under the employment agreement. The employment agreement is at will, meaning either we or Mr. Conley may terminate the employment relationship at any time, with or without cause, upon written notice to the other party. The employment agreement provides for severance pay equal to 12 months of then-in-effect base salary if Mr. Conley is terminated without “cause” or voluntarily terminates his employment for “good reason,” as defined in the employment agreement.
2021 Equity Incentive Plan
On August 12, 2021, we established the Know Labs, Inc. 2021 Equity Incentive Plan (the “2021 Plan”), pursuant to which we may grant incentive stock options, non-qualified stock options, stock appreciation rights, restricted awards, performance share awards, and performance compensation awards to our employees, including the NEOs, officers, consultants and directors, which may be subject to time-based vesting, performance-based vesting or other criteria as determined by the compensation committee of the Board, in accordance with the 2021 Plan. Awards under the 2021 Plan allow eligible participants to participate in the possibility of future value of the Company, depending on the long-term price appreciation of our common stock and the participant’s continuing service with our Company and allow our Company to attract, retain and motivate talent through means of appropriate incentivization to achieve long-range goals and further the alignment of their interests with those of our stockholders.
In Fiscal 2022, we granted stock option awards to our named executive officers, each of which vest quarterly over four years subject to the applicable named executive officer’s continued employment, except that none of Mr. Conley’s stock options may vest within the first six months following the date of grant. All stock options granted in Fiscal 2022 expire on the fifth anniversary of the grant date.
Other Compensation
As compensation for the development of the NFT sales, Mr. Bosua was paid $1,097,928 in compensation and $91,500 for rent expense during the year ended September 30, 2022. See the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Digital Asset Sales” and Note 4 to the Notes to our Consolidated Financial Statements, each included in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, for more information about NFT sales.
| 65 |
| Table of Contents |
Outstanding Equity Awards at Fiscal Year-End
The following table includes certain information with respect to the value of all unexercised options and unvested shares of restricted stock previously awarded to the executive officers named above at the fiscal year ended September 30, 2022.
|
|
| Option Awards | |||||||||||||
|
|
| Number of |
|
| Number of |
|
|
|
|
|
| ||||
|
|
| Securities |
|
| Securities |
|
|
|
|
|
| ||||
|
|
| Underlying |
|
| Underlying |
|
|
|
|
|
| ||||
|
|
| Unexercised |
|
| Unexercised |
|
| Option |
|
|
| ||||
|
|
| Options |
|
| Options |
|
| Exercise |
|
| Option | ||||
|
|
| Exercisable |
|
| Unexerciseable |
|
| Price |
|
| Expiration | ||||
| Name |
| (#) |
|
| (#) |
|
| ($) (4) |
|
| Date | ||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
| Ronald P. Erickson (1) |
|
| 1,200,000 |
|
|
| - |
|
| $ | 1.10 |
|
| 11/4/2024 | |
|
|
|
| - |
|
|
| 1,865,675 |
|
| $ | 1.53 |
|
| 12/15/2025 | |
|
|
|
| 266,525 |
|
|
| 1,599,150 |
|
| $ | 1.53 |
|
| 12/15/2025 | |
|
|
|
| 2,000,000 |
|
|
| - |
|
| $ | 1.53 |
|
| 12/15/2025 | |
|
|
|
| 187,500 |
|
|
| 812,500 |
|
| $ | 2.09 |
|
| 12/16/2026 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Phillip A. Bosua (2) |
|
| 1,000,000 |
|
|
| - |
|
| $ | 1.28 |
|
| 7/30/2023 | |
|
|
|
| - |
|
|
| 1,200,000 |
|
| $ | 1.10 |
|
| 11/4/2024 | |
|
|
|
| - |
|
|
| 2,132,195 |
|
| $ | 1.53 |
|
| 12/15/2025 | |
|
|
|
| 304,600 |
|
|
| 1,827,600 |
|
| $ | 1.53 |
|
| 12/15/2025 | |
|
|
|
| 243,750 |
|
|
| 1,056,250 |
|
| $ | 2.09 |
|
| 12/16/2026 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Peter J. Conley (3) |
|
| - |
|
|
| 1,000,000 |
|
| $ | 1.48 |
|
| 5/20/2027 | |
(1) On November 4, 2019, we granted a stock option grant to Ronald P. Erickson for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon uplisting to the NASDAQ or NYSE exchanges. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $1,207,200 during the year ended September 30, 2022. On December 15, 2020, we issued a stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. The grant vests in increments if the market capitalization of our commons stock exceeds for 20 consecutive trading days starting at $100 million to $1 billion. The Company estimated at grant date the fair value of these options at approximately $520,869 which is being amortized over 5 years. As of September 30, 2022, we recorded a cumulative expense of $186,657. We are valuing this stock option using the Monte Carlo pricing model which included key assumptions of 100% stock volatility, five year life and no forfeitures. The stock option grant was not vested as of September 30, 2022. On December 15, 2020, we issued an additional stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $263,593 during the year ended September 30, 2022. The stock option grants vest when earned based on certain performance criteria. On December 15, 2020, we issued a fully vested warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is exercisable for cash or non-cash at $1.53 per share and was valued using a Black-Scholes model at $1,811,691. On December 16, 2021, we issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
(2) On July 30, 2018, Mr. Bosua was awarded a stock option grant for 1,000,000 shares of our common stock that was awarded at $1.28 per share. The stock option grant vests quarterly over four years. The performance grant was not earned as of September 30, 2022. On November 4, 2019, we granted a stock option grant to Philip A. Bosua for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon FDA approval of the UBAND blood glucose monitor. On December 15, 2020, we issued a stock option grant to Phillip A. Bosua for 2,132,200 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. The grant vests in increments if the market capitalization of our commons stock exceeds for 20 consecutive trading days starting at $100 million to $1 billion. The Company estimated at grant date the fair value of these options at approximately $595,277 which is being amortized over 5 years. As of September 30, 2022, we recorded a cumulative expense of $231,321. We are valuing this stock option using the Monte Carlo pricing model which included key assumptions of 100% stock volatility, five year life and no forfeitures. The stock option grant was not vested as of September 30, 2022. On December 15, 2020, we issued another stock option grant to Phillip A. Bosua for 2,132,195 shares at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $301,249 during the year ended September 30, 2022. On December 16, 2021, we issued a stock option grant to Phillip A. Bosua for 1,300,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
| 66 |
| Table of Contents |
Mr. Bosua resigned from the Board of Directors and from his position as Chief Executive Officer on January 23, 2023. Pursuant to the Separation Agreement, as of January 23, 2023, any of Mr. Bosua’s outstanding stock options ceased vesting as of January 23, 2023, and his vested stock options will remain exercisable until January 23, 2024. However, since the Separation Agreement was executed in the fiscal year ending September 30, 2023, these changes are not reflected in this table.
(3) Mr. Peter J. Conley has served as our Chief Financial Officer and SVP Intellectual Property since May 2022. On May 20, 2022, we issued a stock option grant to Mr. Conley for 1,000,000 shares at an exercise price of $1.48 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years, with no vesting during the first six months.
Additional Narrative Disclosure
Retirement Benefits
We have not maintained, and do not currently maintain, a defined benefit pension plan, nonqualified deferred compensation plan or other similar benefits.
We maintain a 401(k) plan and/or other health and welfare benefit plans in which our NEOs are eligible to participate.
Potential Payments upon Termination or Change in Control
We have the following potential payments upon termination or change in control with Ronald P. Erickson:
|
|
|
|
| Early |
|
| Not For Good |
|
| Change in |
|
|
|
|||||||
| Executive |
| For Cause |
|
| or Normal |
|
| Cause |
|
| Control |
|
| Disability |
|
|||||
| Payments Upon |
| Termination |
|
| Retirement |
|
| Termination |
|
| Termination |
|
| or Death |
|
|||||
| Separation |
| on 9/30/2022 |
|
| on 9/30/2022 |
|
| on 9/30/2022 |
|
| on 9/30/2022 |
|
| on 9/30/2022 |
|
|||||
| Compensation: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
| Base salary (1) |
| $ | - |
|
| $ | - |
|
| $ | 325,000 |
|
| $ | 325,000 |
|
| $ | - |
|
| Performance-based incentive |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| compensation |
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| Stock options (2) |
| $ | - |
|
| $ | - |
|
| $ | 4,618,649 |
|
| $ | 4,618,649 |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Benefits and Perquisites: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Health and welfare benefits (3) |
| $ | - |
|
| $ | - |
|
| $ | 25,524 |
|
| $ | 25,524 |
|
| $ | - |
|
| Accrued vacation pay |
| $ | - |
|
| $ | - |
|
| $ | 40,385 |
|
| $ | 40,385 |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
| $ | - |
|
| $ | - |
|
| $ | 5,009,558 |
|
| $ | 5,009,558 |
|
| $ | - |
|
(1) Reflects a salary for twelve months.
(2) Reflects the vesting of stock option grants.
(3) Reflects the cost of medical benefits for eighteen months.
| 67 |
| Table of Contents |
We have the following potential payments upon termination or change in control with Peter J. Conley:
| Early | Not For Good | Change in | ||||||||||||||||||
| Executive | For Cause | or Normal | Cause | Control | Disability | |||||||||||||||
| Payments Upon | Termination | Retirement | Termination | Termination | or Death | |||||||||||||||
| Separation | on 9/30/2022 | on 9/30/2022 | on 9/30/2022 | on 9/30/2022 | on 9/30/2022 | |||||||||||||||
| Compensation: | ||||||||||||||||||||
| Base salary (1) | $ | - | $ | - | $ | 300,000 | $ | 300,000 | $ | - | ||||||||||
| Performance-based incentive | ||||||||||||||||||||
| compensation | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
| Stock options (2) | $ | - | $ | - | $ | 979,000 | $ | 979,000 | $ | - | ||||||||||
| Benefits and Perquisites: | ||||||||||||||||||||
| Health and welfare benefits | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
| Accrued vacation pay | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
| Total | $ | - | $ | - | $ | 1,279,000 | $ | 1,279,000 | $ | - | ||||||||||
(1) Reflects a salary for twelve months.
(2) Reflects the vesting of stock option grants.
On January 23, 2023, Mr. Bosua resigned from his position as Chief Executive Officer of the Company. Pursuant to his Separation Agreement, Mr. Bosua received a cash payment of $400,000 and an additional payment of $13,806, representing continued medical benefits for a period of eighteen months. Mr. Bosua will also receive $10,000 per month while serving as a consultant for the Company through January 23, 2024. In connection with the Separation Agreement, Mr. Bosua’s Employment Agreement was terminated and as a result he is no longer entitled to any other payments upon termination or change in control.
| 68 |
| Table of Contents |
Our independent non-employee directors are primarily compensated with stock option grants and stock grants to attract and retain qualified candidates to serve on the Board, in addition to a $10,000 cash retainer in consideration of board services. In setting director compensation, we consider the significant amount of time that directors expend in fulfilling their duties to our Company as well as the skill-level required by our members of the Board.
The table below sets forth the compensation paid to our non-employee directors during the fiscal year ended September 30, 2022. Ronald P. Erickson and Phillip A. Bosua did not receive any compensation for their services as directors. The compensation disclosed in the Summary Compensation Table above represents the total compensation for Mr. Erickson and Mr. Bosua. Mr. Bosua resigned from the Board on January 23, 2023.
|
|
| Stock |
|
| Option |
|
| Fees |
|
|
|
|
||||
| Name |
| Awards (4) |
|
| Awards (4) |
|
| Earned |
|
| Total |
|
||||
| Jon Pepper (1) |
| $ | 51,000 |
|
| $ | 23,740 |
|
| $ | 10,000 |
|
| $ | 84,740 |
|
| Ichiro Takesako (2) |
|
| 51,000 |
|
|
| 23,740 |
|
|
| 10,000 |
|
|
| 84,740 |
|
| William A. Owens (3) |
|
| 51,000 |
|
|
| 23,740 |
|
|
| 10,000 |
|
|
| 84,740 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
| $ | 153,000 |
|
| $ | 71,220 |
|
| $ | 30,000 |
|
| $ | 254,220 |
|
(1) The stock award for 30,000 shares was issued on January 5, 2022 to Jon Pepper and was valued at $1.70 per share. The stock option grant for 20,000 shares of common stock was issued on January 5, 2022 to Mr. Pepper and was valued at the black scholes value of $1.187 per share. Mr. Pepper was paid $10,000 for board services. As of June 30, 2023, Mr. Pepper has stock option grants for 77,500 shares of common stock and warrants to purchase common stock of 65,000 shares.
(2) The stock award for 30,000 shares was issued on January 5, 2022 to Ichiro Takesako and was valued at $1.70 per share. The stock option grant for 20,000 shares of common stock was issued on January 5, 2022 to Mr. Takesako and was valued at the black scholes value of $1.187 per share. Mr. Takesako was paid $10,000 for board services. As of June 30, 2023, Mr. Takesako has stock option grants for 77,500 shares of common stock and warrants to purchase common stock of 40,000 shares.
(3) The stock award for 30,000 shares was issued on January 5, 2022 to William A. Owens and was valued at $1.70 per share. The stock option grant for 20,000 shares of common stock was issued on January 5, 2022 to Mr. Owens and was valued at the black scholes value of $1.187 per share. Mr. Owens was paid $10,000 for board services. As of June 30, 2023, Mr. Owens has warrants to purchase common stock of 271,250 shares.
(4) These amounts reflect the aggregate grant date fair value of awards granted in the fiscal year ended September 30, 2022, as required by Regulation S-K Item 402(n)(2), computed in accordance with the FASB ASC Topic 718. All assumptions made in the valuations are contained and described in footnote 10 to the Company’s financial statements for Fiscal 2022 contained in our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, filed with the SEC on December 20, 2022. The amounts shown in the table reflect the total fair value on the date of grant and do not necessarily reflect the actual value, if any, that may be realized by the listed executives.
| 69 |
| Table of Contents |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transactions with Related Persons
The following includes a summary of transactions since the beginning of our 2021 fiscal year, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last three completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation described under “Executive Compensation” above). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
Transactions with Clayton Struve
On May 3, 2022, we approved the Extension of Warrant Agreement with Clayton Struve, extending the exercise dates as follows:
| Warrant No./Class |
| Issue Date |
| No. Warrant Shares |
|
| Exercise Price |
|
| Original Expiration Date |
| Amended Expiration Date | |||
| Clayton A. Struve Warrant |
| 08-14-2017 |
|
| 1,440,000 |
|
| $ | 0.25 |
|
| 08-13-2023 |
| 08-13-2024 | |
| Clayton A. Struve Warrant |
| 12-12-2017 |
|
| 1,200,000 |
|
| $ | 0.25 |
|
| 12-11-2023 |
| 12-11-2024 | |
| Clayton A. Struve Warrant |
| 08-04-2016 |
|
| 1,785,715 |
|
| $ | 0.25 |
|
| 08-04-2023 |
| 08-04-2024 | |
| Clayton A. Struve Warrant |
| 02-28-2018 |
|
| 1,344,000 |
|
| $ | 0.25 |
|
| 02-28-2023 |
| 02-28-2024 | |
On January 28, 2021, Clayton A. Struve exercised warrants on a cashless basis for 889,880 shares of common stock at $0.25 per share, including warrants for 187,500 and 187,500 that were previously extended. We recorded interest expense of $244,260 during the year ended September 30, 2021 related to the extension of the warrants.
On December 7, 2022, we signed an Extension of Warrant Agreement with Clayton Struve, extending the exercise dates as follows:
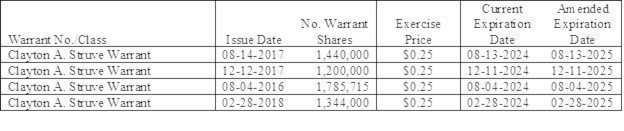
We recorded interest expense of $194,019 during the nine months ended June 30, 2023 related to the extension of the warrants. We recorded the original value of warrants in equity and as such, we recorded the extension value as an expense with an offset to additional paid in capital.
| 70 |
| Table of Contents |
Convertible Promissory Notes with Clayton A. Struve
We owe Clayton A. Struve, a significant stockholder, $1,071,000 under convertible promissory or OID notes. We recorded accrued interest of $92,171 and $86,562 as of June 30, 2023 and September 30, 2022, respectively. On December 7, 2022, we signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2023. We expensed $155,702 during the nine months ended June 30, 2023 related to the extension of the notes. We recorded in equity the incremental value related to the conversion feature and as such, we recorded the extension value as an expense with an offset to additional paid in capital.
Series C and D Preferred Stock and Warrants
On August 5, 2016, we closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve for the purchase of $1,250,000 of preferred stock with a conversion price of $0.70 per share. The preferred stock has a yield of 8% and an ownership blocker of 4.99%. In addition, Mr. Struve received a five-year warrant to acquire 1,785,714 shares of common stock at $0.70 per share. On August 14, 2017, the conversion price of the Series C Preferred Stock was adjusted to $0.25 per share pursuant to its certificate of designation. As of June 30, 2023, Mr. Struve owns all of the 1,785,715 issued and outstanding shares of Series C Preferred Stock.
As of June 30, 2023, Mr. Struve owns all of the 1,016,004 issued and outstanding shares of Series D Preferred Stock
On June 28, 2023, Mr. Struve converted $350,696 of accumulated Series D preferred stock dividends into 1,402,784 shares of our common stock.
On August 9, 2023, the Board authorized the Company to file a series of amendments to certain classes of preferred stock, the certificates of designation, and restatement of its articles of incorporation, as described below, each of which were filed with the Nevada Secretary of State effective August 11, 2023. See Item 5. Based upon the modified terms and conditions of Series C and D certificates of designations, it was determined that Series C and D preferred dividends need to be accreted going forward. As of June 30, 2023, cumulative unpaid Series C and D totaled approximately $730,000 which converts to approximately 2,920,000 shares of common stock. The value of the 2.9 million shares of common totaled $3,337,494. The Company recorded $3,337,494 in cumulative deemed dividends related to Series C and D Preferred Stock which have not been paid.
See “Description of Securities” for the terms of our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock.
Debt Offering
Mr. Struve invested $1,000,000 in our debt offering which closed in May 2019. On March 18, 2020, Mr. Struve received 1,080,000 shares of common stock related to the automatic conversion of the $1,000,000 invested in the debt offering.
Transactions with Ronald P. Erickson
On March 16, 2018, we entered into a Note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 currently owing under the J3E2A2Z Notes was converted to a Convertible Redeemable Promissory Note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z Account Payable was converted into a Convertible Redeemable Promissory Note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of our common stock for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. We recorded accrued interest of $320,427 and $287,290 as of June 30, 2023 and September 30, 2022, respectively. On December 7, 2022, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to January 30, 2023. On January 25, 2023, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to September 30, 2023. Mr. Erickson controls J3JE2A2Z.
On November 4, 2019, we granted a stock option grant to Ronald P. Erickson for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon uplisting to the NASDAQ or NYSE exchanges. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $1,207,200 during the year ended September 30, 2022.
On June 1, 2020, Mr. Erickson received a salary of $10,000 per month for work on Particle, Inc. This salary was cancelled as of August 15, 2021.
| 71 |
| Table of Contents |
On July 2, 2020, Particle issued a stock option grant for 1,500,000 shares at $0.10 per share to Ronald P. Erickson. The stock option grant vests (i) 33.3% upon issuance; (ii) 33.3% after the first sale; and (iii) 33.4% after one million in sales are achieved. The stock option grant was forfeited as of September 30, 2021.
On December 15, 2020, we issued a stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. The grant vests in increments if the market capitalization of our commons stock exceeds for 20 consecutive trading days starting at $100 million to $1 billion. We estimated at grant date the fair value of these options at approximately $520,869 which is being amortized over 5 years. As of September 30, 2022, we recorded a cumulative expense of $186,657. We are valuing this stock option using the Monte Carlo pricing model which included key assumptions of 100% stock volatility, five year life and no forfeitures. The stock option grant was not vested as of September 30, 2022.
On December 15, 2020, we issued a stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $263,593 during the year ended September 30, 2022. The stock option grants vest when earned based on certain performance criteria.
On December 15, 2020, we issued warrants to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is immediately vested and exercisable on a cash or cashless basis at $1.53 per share and was valued using a Black-Scholes model at $1,811,691.
On December 16, 2021, we issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On December 14, 2022, we issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $1.41 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On January 19, 2023, we signed an Extension of Warrant Agreements with Ronald P. Erickson and an entity controlled by Mr. Erickson, extending the exercise dates from January 30, 2023 to January 30, 2024.
Mr. Erickson and/or entities with which he is affiliated also have expenses and interest of approximately $322,715 as of March 31, 2023, respectively.
Transactions with Peter J. Conley
On May 20, 2022, we issued a stock option grant to Mr. Conley for 1,000,000 shares at an exercise price of $1.48 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years, with no vesting during the first six months.
Stock Issuances to Named Executive Officers and Directors
On January 15, 2021, we issued 30,000 shares each to three directors shares at an exercise price of $2.00 per share.
On January 15, 2021, we issued 20,000 warrants to purchase common stock each to three directors shares at $2.00 per share. The warrants expire on January 15, 2026.
On January 5, 2022, we issued 30,000 shares each to three directors shares at an exercise price of $1.70 per share.
On January 5, 2022, we issued 20,000 warrants to purchase common stock each to three directors shares at $1.70 per share. The warrants expire on January 5, 2027.
On February 15, 2023, we issued stock option grants to two directors for a total of 50,000 shares at an exercise price of $1.24 per share. The stock option grant expires in five years. The stock option grants vested at issuance.
| 72 |
| Table of Contents |
Indemnification
Our articles of incorporation provide that we will indemnify our directors and officers to the fullest extent permitted by Nevada law. In addition, we have Indemnification Agreements with the current Board of Directors.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of September , 2023 for (i) each of our named executive officers and directors; (ii) all of our named executive officers and directors as a group; and (iii) each other stockholder known by us to be the beneficial owner of more than 5% of our outstanding common stock. Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o our company, 500 Union Street, Suite 810, Seattle, WA 98101.
|
|
| Shares Beneficially Owned (1) (2) |
|
|||||
| Name of Beneficial Owner |
| Amount |
|
| Percentage |
|
||
| Directors and Officers- |
|
|
|
|
|
|
||
| Ronald P. Erickson (3) |
|
| 12,085,540 |
|
|
| 19.2 | % |
| Peter J. Conley (4) |
|
| 260,000 |
|
|
| 0.5 | % |
| Jon Pepper (5) |
|
| 535,500 |
|
|
| 1.0 | % |
| Ichiro Takesako (6) |
|
| 137,500 |
|
|
| 0.3 | % |
| William A. Owens (7) |
|
| 1,083,953 |
|
|
| 2.1 | % |
| All executive officers and directors (5 persons) |
|
| 14,102,493 |
|
|
| 13.7 | % |
* Less than 1%
|
| (1) | Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares that such person or any member of such group has the right to acquire within sixty (60) days. For purposes of computing the percentage of outstanding shares of our common stock held by each person or group of persons named above, any shares that such person or persons has the right to acquire within sixty (60) days of September , 2023 are deemed to be outstanding for such person, but not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares listed as beneficially owned does not constitute an admission of beneficial ownership by any person. |
|
|
|
|
|
| (2) | Based on 52,358,463 shares of common stock issued and outstanding as of September , 2023. |
|
|
|
|
|
| (3) | Consists of (i) 1,488,085 shares of shares of our common stock beneficially owned by Ronald P. Erickson or entities controlled by Mr. Erickson; (ii) 1,966,525 shares of our common stock issuable upon the exercise of options exercisable within 60 days; (iii) 3,894,666 shares of our common stock issuable upon the exercise of warrants that are exercisable within 60 days; and (iv) 4,736,264 shares of our common stock issuable upon the conversion of convertible debt that is convertible within 60 days. |
|
|
|
|
|
| (4) | Consists of (i) 10,000 shares of our common stock held directly by Peter Conley and (ii) 250,000 shares of our common stock issuable upon the exercise of options exercisable within 60 days. |
|
|
|
|
|
| (5) | Consists of (i) 393,000 shares of our common stock held directly by Jon Pepper, (ii) 77,500 shares of our common stock issuable upon the exercise of options exercisable within 60 days and (iii) 65,000 shares of our common stock issuable upon the exercise of warrants exercisable within 60 days. |
|
|
|
|
|
| (6) | Consists of (i) 20,000 shares of our common stock held directly by Ichiro Takesako, (ii) 77,500 shares of our common stock issuable upon the exercise of options exercisable within 60 days and (iii) 40,000 shares of our common stock issuable upon the exercise of warrants exercisable within 60 days. |
|
|
|
|
|
| (7) | Consists of (i) 812,703 shares of our common stock held directly by William A Owens and (ii) 271,250 shares of our common stock issuable upon the exercise of warrants that are exercisable within 60 days. |
| 73 |
| Table of Contents |
|
|
| Shares Beneficially Owned |
|
|||||
|
|
| Amount |
|
| Percentage |
|
||
| Greater Than 5% Ownership |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||
| Clayton A. Struve(1) |
|
| 20,064,855 |
|
|
| 28.3 | % |
|
|
| Blocker at 4.99 | % |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
| Ronald P. Erickson(2) |
|
| 12,085,540 |
|
|
| 19.2 | % |
|
|
|
|
|
|
|
|
|
|
| Todd Baszucki(3) |
|
| 5,583,000 |
|
|
| 10.5 | % |
|
|
|
|
|
|
|
|
|
|
| Phillip A. Bosua(4) |
|
| 4,634,600 |
|
|
| 8.6 | % |
|
| (1) | Beneficial ownership includes 1,402,784 shares of our common stock and 6,269,715 shares of our common stock issuable upon the exercise of warrants, 8,108,356 shares of our common stock issuable upon the conversion of our Series C Convertible Preferred Stock and our Series D Convertible Preferred Stock and 4,284,000 shares of our common stock issuable upon the conversion of convertible notes. All of the warrants, Series C Convertible Preferred Stock, Series D Convertible Preferred Stock and convertible notes held by Mr. Struve are subject to a 4.99% blocker pursuant to which shares of our common stock may not be issued to the extent that such issuance would cause Mr. Struve to beneficially own more than 4.99% of our common stock. The address of Mr. Struve is 175 West Jackson Blvd., Suite 440, Chicago, IL 60604. |
|
|
|
|
|
| (2) | See above for Ronald P. Erickson or entities controlled by Mr. Erickson. |
|
|
|
|
|
| (3) | Includes (i) 4,583,000 shares of our common stock held directly by Todd Baszucki and (ii) 1,000,000 shares of our common stock issuable upon the exercise of warrants. The address for Mr. Baszucki is 395 Del Monte Center, Unit 306, Monterey, CA 93940. |
|
|
|
|
|
| (4) | Consists of (i) 3,005,000 shares of shares of our common stock held directly by Phillip A. Bosua and (ii) 1,629,600 shares of our common stock issuable upon the exercise of options that are exercisable within 60 days. The address for Mr. Bosua is 201 Galer, Unit 410, Seattle WA 98109. Mr. Bosua resigned from the Board of Directors and from his position as Chief Executive Officer on January 23, 2023. Mr. Bosua is party to a Separation and Release Agreement with the Company, pursuant to which he was entitled to receive severance payments. Such payments are described in greater detail below under “Employment and Separation Agreements” and such amounts will be disclosed in the summary compensation table for the fiscal year ended September 30, 2023. |
| 74 |
| Table of Contents |
The following description summarizes certain terms of our capital stock and the securities being sold in this offering. Because this is a summary description, it does not contain all of the information that may be important to you. This summary does not purport to be complete and is qualified in its entirety by the provisions of our articles of incorporation as amended, restated and supplemented to date, or our articles of incorporation, and our second amended and restated bylaws, or our bylaws, which have been filed as exhibits to the registration statement of which this prospectus is a part, as well as the applicable provisions of the Nevada Revised Statutes.
General
Authorized Capital Stock. Our authorized capital stock currently consists of:
|
| · | 200,000,000 shares of common stock, par value $0.001 per share; and |
|
|
|
|
|
| · | 5,000,000 shares of “blank check” preferred stock, par value $0.001 per share, of which: |
|
| · | 30,000 shares have been designated as our Series C Convertible Preferred Stock, $0.001 par value per share; and |
|
|
|
|
|
| · | 20,000 shares have been designated as our Series D Convertible Preferred Stock, $0.001 par value per share. |
Outstanding Shares of Capital Stock. Our common stock is the only security of the Company registered pursuant to Section 12 of the Securities Exchange Act of 1934, as amended. All outstanding shares of our capital stock are fully paid and nonassessable. As of the date of this prospectus, there were:
|
| · | 52,358,463 shares of common stock issued and outstanding, held by holders of record; |
|
|
|
|
|
| · | 17,858 shares of Series C Convertible Preferred Stock issued and outstanding, held by one holder of record; and |
|
|
|
|
|
| · | 10,161 shares of Series D Convertible Preferred Stock issued and outstanding, held by one holder of record. |
Common Stock
Holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors. Our articles of incorporation do not provide for cumulative voting in the election of directors.
Subject to any preferential rights of any outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the board of directors on the common stock out of legally available funds. In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any preferential rights of any outstanding preferred stock.
Holders of common stock have no preemptive, conversion or subscription rights and there are no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock, including our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock.
Preferred Stock
Our articles of incorporation authorize our board of directors, without stockholder approval, to issue up to 5,000,000 shares of preferred stock in one or more series, and to determine the designation, preferences, limitations and relative rights thereof, including, without limitation, such matters as dividends, redemption, liquidation, conversion and voting. Our board of directors has the discretion to issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of common stock, or which could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
Series C Convertible Preferred Stock
Of our authorized preferred stock, 30,000 shares have been designated as our Series C Convertible Preferred Stock, or the Series C Preferred Stock.
| 75 |
| Table of Contents |
With respect to dividend rights and rights on liquidation, winding up and dissolution, shares of our Series C Preferred stock rank senior to our common stock and our Series D Convertible Preferred Stock. Holders of Series C Preferred Stock have no preemptive or subscription rights and there are no redemption or sinking fund provisions applicable to the Series C Preferred Stock. The rights, preferences and privileges of the holders of Series C Preferred Stock are subject to, and may be adversely affected by, the rights of the holders of shares of any other series of preferred stock.
In addition to any class voting rights provided by the Nevada Revised Statutes or the certificate of designation for the Series C Preferred Stock, holders of Series C Preferred Stock have the right to vote, on an as-if-converted-to-common-stock basis (but subject to, and after giving effect to, the conversion limitations described below, applied effective as of the record date for determining the stockholders entitled to vote). Further, as long as any shares of Series C Preferred are outstanding, the Company shall not, among other things, without the affirmative vote of the holders of at least a majority on voting power of the outstanding shares of Series C Preferred Stock: (a) alter or change adversely the powers, preferences or rights given to the Series C Preferred Stock or alter or amend the Series C Preferred Stock certificate of designation, (b) issue any other class or series of capital stock ranking senior to or on parity the Series C Preferred Stock as to dividends or up liquidation or reclassify any shares of common stock or any series of capital stock into shares having preference or priority as to dividends or upon liquidation superior to or on parity with any such preference or priority of Series C Preferred Stock, or (c) enter into any agreement with respect to any of the foregoing.
Each outstanding share of Series C Preferred Stock accrues cumulative dividends at a rate equal to 8.0% per annum of the Series C Preferred Stock stated value (currently $70.00, subject to adjustment as provided in the Series C Preferred Stock certificate of designation). Dividends, whether accrued, declared or payable are payable solely in the form of additional shares of Series C Preferred Stock and shall not in any circumstances be accrued or payable in cash. Such dividends are payable only upon conversion of the shares of Series C Preferred Stock, or when, as and if otherwise declared by our board of directors.
Each holder of any shares of Series C Preferred Stock has the right, at its option at any time, to convert such holder’s shares of Series C Preferred Stock into shares of our common stock in accordance with the terms of the Series C Preferred Stock certificate of designation. Further, we may also require, upon notice, the conversion of any or all shares of the Series C Preferred Stock into our common stock provided that the shares issuable upon such conversion meet certain resale eligibility requirements, and our common stock has been approved for listing on specified stock exchanges, all as set forth in the Series C Preferred Stock certificate of designation. However, we shall not effect a conversion of the Series C Preferred Stock, whether voluntary or mandatory, and the holder of any shares of Series C Preferred Stock shall not have the right to voluntarily convert such holder’s shares of Series C Preferred Stock, to the extent that after giving effect to such exercise, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the shares of our common stock outstanding immediately after giving effect to such conversion. By written notice to the Corporation, a holder may from time to time increase or decrease such percentage to any other percentage not less than 4.99% and not in excess of 9.99% specified in such notice; provided that any such increase or decrease will only be effective for that holder and will not be effective until the 61st day after such notice is delivered to us.
The Series C Preferred Stock also has price-based, “full-ratchet,” and proportional anti-dilution rights, based on issuance or deemed issuances of our securities below the current conversion price of $0.25 per share, all as set forth in the Series C Preferred Stock certificate of designation.
Series D Convertible Preferred Stock
Of our authorized preferred stock, 20,000 shares have been designated as our Series D Convertible Preferred Stock, or the Series D Preferred Stock. With respect to dividend rights and rights on liquidation, winding up and dissolution, shares of our Series D Preferred Stock rank senior to our common stock but junior to our Series C Preferred Stock. Holders of Series D Preferred Stock have no preemptive or subscription rights and there are no redemption or sinking fund provisions applicable to the Series D Preferred Stock. The rights, preferences and privileges of the holders of Series D Preferred Stock are subject to, and may be adversely affected by, the rights of the holders of shares of any other series of preferred stock.
| 76 |
| Table of Contents |
In addition to any class voting rights provided by the Nevada Revised Statutes or the certificate of designation for the Series D Preferred Stock, holders of Series D Preferred Stock have the right to vote, on an as-if-converted-to-common-stock basis (but subject to, and after giving effect to, the conversion limitations described below, applied effective as of the record date for determining the stockholders entitled to vote). Further, as long as any shares of Series D Preferred are outstanding, the Company shall not, among other things, without the affirmative vote of the holders of at least a majority on voting power of the outstanding shares of Series D Preferred Stock: (a) alter or change adversely the powers, preferences or rights given to the Series D Preferred Stock or alter or amend the Series D Preferred Stock certificate of designation, (b) issue any other class or series of capital stock ranking senior to or on parity the Series D Preferred Stock as to dividends or up liquidation or reclassify any shares of common stock or any series of capital stock into shares having preference or priority as to dividends or upon liquidation superior to or on parity with any such preference or priority of Series D Preferred Stock, or (c) enter into any agreement with respect to any of the foregoing.
Each outstanding share of Series D Preferred Stock accrues cumulative dividends at a rate equal to 8.0% per annum of the Series D Preferred Stock stated value (currently $70.00, subject to adjustment as provided in the Series D Preferred Stock certificate of designation). Dividends, whether accrued, declared or payable are payable solely in the form of additional shares of Series D Preferred Stock and shall not in any circumstances be accrued or payable in cash. Such dividends are payable only upon conversion of the shares of Series D Preferred Stock, or when, as and if otherwise declared by our board of directors.
Each holder of any shares of Series D Preferred Stock has the right, at its option at any time, to convert such holder’s shares of Series D Preferred Stock into shares of our common stock in accordance with the terms of the Series D Preferred Stock certificate of designation. Further, we may also require, upon notice, the conversion of any or all shares of the Series D Preferred Stock into our common stock provided that the shares issuable upon such conversion meet certain resale eligibility requirements, and our common stock has been approved for listing on specified stock exchanges, all as set forth in the Series D Preferred Stock certificate of designation. However, we shall not effect a conversion of the Series D Preferred Stock, whether voluntary or mandatory, and the holder of any shares of Series D Preferred Stock shall not have the right to voluntarily convert such holder’s shares of Series D Preferred Stock, to the extent that after giving effect to such exercise, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the shares of our common stock outstanding immediately after giving effect to such conversion. By written notice to the Corporation, a holder may from time to time increase or decrease such percentage to any other percentage not less than 4.99% and not in excess of 9.99% specified in such notice; provided that any such increase or decrease will only be effective for that holder and will not be effective until the 61st day after such notice is delivered to us.
The Series D Preferred Stock also has price-based, “full-ratchet,” and proportional anti-dilution rights, based on issuance or deemed issuances of our securities below the current conversion price of $0.25 per share, all as set forth in the Series D Preferred Stock certificate of designation.
Underwriter’s Warrant to be Issued as Part of this Offering
Upon the closing of this offering, there will be up to [*] shares of common stock upon exercise of the underwriter’s warrant. See “Underwriting” below. Such summary of certain terms and provisions of the underwriter’s warrant is not complete and is subject to, and qualified in its entirety by, the provisions of the underwriter’s warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Existing Warrants
As of the date of this prospectus, we have issued warrants for the purchase of 21,736,313 shares of common stock at a weighted average price of $1.031. The expiration dates of these warrants range from June 30, 2023 to September 26, 2027.
If in the future, we sell common stock at a price below $0.25 per share, the conversion price of our outstanding shares of Series C Convertible Preferred Stock and Series D Convertible Preferred Stock would adjust below $0.25 per share pursuant to their respective certificates of designation. In addition, the conversion price of the convertible promissory notes referred to above and the exercise price of certain outstanding warrants to purchase 7,684,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments. Warrants totaling 4,439,707 would adjust below $1.20 per share and warrants totaling 4,424,425 would adjust below $2.40 per share, in each case pursuant to the documents governing such instruments.
| 77 |
| Table of Contents |
Clayton A. Struve has warrants to purchase 6,269,715 shares of common stock that have a beneficial ownership blocker at 4.99%.
The proceeds of warrants currently outstanding, which could be exercised on a cash basis, may generate potential proceeds of up to $15,682,308 if exercised in full for cash. We cannot guarantee these warrants will be exercised.
Options
There are 14,506,158 (including unearned stock option grants totaling 3,869,825 shares related to performance milestones) options to purchase common stock at an average exercise price of $1.546 per share outstanding as of June 30, 2023 under the 2021 Plan. The expiration dates of these stock options range from June 30, 2023 to March 27, 2028.
Convertible Promissory Notes
We owe Clayton A. Struve, a significant stockholder, $1,071,000 under convertible promissory or OID notes. We recorded accrued interest of $92,171 and $86,562 as of June 30, 2023 and September 30, 2022, respectively. On December 7, 2022, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2023. The Company expensed $155,702 as interest during the nine months ended June 30, 2023 related to the extension of the notes. The Company recorded in equity the incremental value related to the conversion feature and as such, the Company recorded the extension value as an expense with an offset to additional paid in capital.
On March 16, 2018, we entered into a Note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 currently owing under the J3E2A2Z Notes was converted to a Convertible Redeemable Promissory Note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z Account Payable was converted into a Convertible Redeemable Promissory Note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of common stock of our for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. We recorded accrued interest of $320,427 and $287,290 as of June 30, 2023 and September 30, 2022, respectively. On December 7, 2022, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to January 30, 2023. On January 25, 2023, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to September 30, 2023. Mr. Erickson controls J3JE2A2Z.
Anti-Takeover Effects of Certain Provisions of Nevada Law and our Governing Documents
Provisions of the Nevada Revised Statutes, our articles of incorporation and our bylaws could have the effect of delaying or preventing a third-party from acquiring us, even if the acquisition could benefit our stockholders. Such provisions of the Nevada Revised Statutes, our articles of incorporation and our bylaws can have the effect of enhancing continuity and stability in the composition of our board of directors and the policies formulated by the board of directors, and can also have the effect of discouraging certain types of transactions that may involve an actual or threatened change of control of our company. These provisions also may have the effect of reducing our vulnerability to an unsolicited proposal for a takeover that does not contemplate the acquisition of all of our outstanding shares, or an unsolicited proposal for the restructuring or sale of all or part of our company.
| 78 |
| Table of Contents |
Nevada Anti-Takeover Statutes
The Nevada Revised Statutes, or NRS, contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. Nevada’s “acquisition of controlling interest” statutes (NRS 78.378 through 78.3793, inclusive) contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. These “control share” laws provide generally that any person that acquires a “controlling interest” in certain Nevada corporations may be denied voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. These laws will apply to us as of a particular date if we were to have 200 or more stockholders of record (at least 100 of whom have addresses in Nevada appearing on our stock ledger at all times during the 90 days immediately preceding that date) and do business in the State of Nevada directly or through an affiliated corporation, unless our articles of incorporation or bylaws in effect on the tenth day after the acquisition of a controlling interest provide otherwise. These laws provide that a person acquires a “controlling interest” whenever a person acquires shares of a subject corporation that, but for the application of these provisions of the NRS, would enable that person to exercise (1) one-fifth or more, but less than one-third, (2) one-third or more, but less than a majority or (3) a majority or more, of all of the voting power of the corporation in the election of directors. Once an acquirer crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold and within the 90 days immediately preceding the date when the acquiring person acquired or offered to acquire a controlling interest become “control shares” to which the voting restrictions described above apply. These laws may have a chilling effect on certain transactions if our articles of incorporation or bylaws are not amended to provide that these provisions do not apply to us or to an acquisition of a controlling interest, or if our disinterested stockholders do not confer voting rights in the control shares.
Nevada’s “combinations with interested stockholders” statutes (NRS 78.411 through 78.444, inclusive) provide that specified types of business “combinations” between certain Nevada corporations and any person deemed to be an “interested stockholder” of the corporation are prohibited for two years after such person first becomes an “interested stockholder” unless the corporation’s board of directors approves the combination (or the transaction by which such person becomes an “interested stockholder”) in advance, or unless the combination is approved by the board of directors and sixty percent of the corporation’s voting power not beneficially owned by the interested stockholder, its affiliates and associates. Furthermore, in the absence of prior approval certain restrictions may apply even after such two-year period. For purposes of these statutes, an “interested stockholder” is any person who is (1) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting shares of the corporation, or (2) an affiliate or associate of the corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then-outstanding shares of the corporation. The definition of the term “combination” is sufficiently broad to cover most significant transactions between a corporation and an “interested stockholder”. These laws generally apply to Nevada corporations with 200 or more stockholders of record. However, a Nevada corporation may elect in its articles of incorporation not to be governed by these particular laws, but if such election is not made in the corporation’s original articles of incorporation, the amendment (1) must be approved by the affirmative vote of the holders of stock representing a majority of the outstanding voting power of the corporation not beneficially owned by interested stockholders or their affiliates and associates, and (2) is not effective until 18 months after the vote approving the amendment and does not apply to any combination with a person who first became an interested stockholder on or before the effective date of the amendment. Neither our original articles of incorporation nor our current articles of incorporation include such an election.
NRS 78.139 also provides that directors may resist a change or potential change in control of the corporation if the board of directors determines that the change or potential change is opposed to or not in the best interest of the corporation upon consideration of any relevant facts, circumstances, contingencies or constituencies pursuant to NRS 78.138(4). The Nevada Revised Statutes also provide that any director may be removed from our board of directors by the vote or written consent of stockholders representing not less than two-thirds of the voting power of the issued and outstanding shares entitled to vote, and this standard is also reflected in our bylaws.
Bylaws
Our bylaws contain limitations as to who may call special meetings and also establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors.
Authorized but Unissued Shares
Our authorized but unissued shares of common stock are available for our board of directors to issue without stockholder approval. We may use these additional shares for a variety of corporate purposes, including future public or private offerings to raise additional capital, corporate acquisitions and employee benefit plans. The existence of our authorized but unissued shares of common stock could render more difficult or discourage an attempt to obtain control of our company by means of a proxy contest, tender offer, merger or other transaction. Our authorized but unissued shares may be used to delay, defer or prevent a tender offer or takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares held by our stockholders.
| 79 |
| Table of Contents |
Listing
Our common stock is listed on the NYSE American stock exchange under the symbol “KNW.”
Transfer Agent and Registrar
We have appointed Equiniti Trust Company located at 6201 15th Avenue, Brooklyn, New York 11219, telephone number (800) 937-5449, as the transfer agent for our common stock.
| 80 |
| Table of Contents |
MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS FOR NON‑U.S. HOLDERS OF OUR COMMON STOCK
The following is a summary of the material U.S. federal income and estate tax considerations relating to the purchase, ownership and disposition of our common stock that is being issued pursuant to this offering. This summary is limited to Non‑U.S. Holders (as defined below) that hold our common stock as a capital asset (generally, property held for investment) for U.S. federal income tax purposes. This summary does not discuss all of the aspects of U.S. federal income and estate taxation that may be relevant to a Non‑U.S. Holder in light of the Non‑U.S. Holder’s particular investment or other circumstances. Accordingly, all prospective Non‑U.S. Holders should consult their own tax advisors with respect to the U.S. federal, state, local and non‑U.S. tax consequences of the ownership and disposition of our common stock.
This summary is based on provisions of the Code, applicable U.S. Treasury regulations promulgated or proposed thereunder and administrative and judicial interpretations thereof, all as in effect or in existence on the date of this prospectus. Subsequent developments in U.S. federal income or estate tax law, including changes in law or differing interpretations, which may be applied retroactively, could alter the U.S. federal income and estate tax consequences of owning and disposing of our common stock as described in this summary. There can be no assurance that the Internal Revenue Service, or IRS, will not take a contrary position with respect to one or more of the tax consequences described herein and we have not obtained, nor do we intend to obtain, a ruling from the IRS with respect to the U.S. federal income or estate tax consequences of the ownership or disposition of our common stock.
As used in this summary, the term “Non‑U.S. Holder” means a beneficial owner of our common stock that is not, for U.S. federal income tax purposes:
|
| · | an individual who is a citizen or resident of the United States; |
|
|
|
|
|
| · | a corporation (or other entity treated as a corporation) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
|
|
|
|
|
| · | an entity or arrangement treated as a partnership; |
|
|
|
|
|
| · | an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or a trust, if (1) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more “United States persons” (within the meaning of the Code) has the authority to control all of the trust’s substantial decisions, or (2) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a United States person. |
|
|
|
|
| 81 |
| Table of Contents |
An individual may be a resident alien if the individual is a lawful permanent resident of the United States (e.g., a green card holder) and may, in many cases, be deemed to be a resident alien, as opposed to a nonresident alien, by virtue of being present in the United States for at least 31 days in the calendar year and for an aggregate of at least 183 days during a three-year period ending in and including the current calendar year. For these purposes, all the days present in the United States in the current year, one-third of the days present in the immediately preceding year, and one-sixth of the days present in the second preceding year are counted. Resident aliens are subject to U.S. federal income tax as if they are U.S. citizens. Such an individual is urged to consult his or her own tax advisor regarding the U.S. federal income and estate tax consequences of the purchase, ownership or disposition of our common stock.
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in such a partnership generally will depend upon the status of the partner, the activities of the partnership and certain determinations made at the partner level. Partnerships that hold our common stock and partners in such partnerships should consult their own tax advisors as to the particular U.S. federal income and estate tax consequences of owning and disposing of our common stock that are applicable to them.
This summary does not consider any specific facts or circumstances that may apply to a Non‑U.S. Holder and does not address any special tax rules that may apply to particular Non‑U.S. Holders, such as:
|
| · | a Non‑U.S. Holder that is a financial institution, insurance company, tax‑exempt organization, pension plan, broker, dealer or trader in securities, dealer in currencies, U.S. expatriate, controlled foreign corporation or passive foreign investment company; |
|
|
|
|
|
| · | a Non-U.S. Holder that is subject to special accounting rules under Section 451(b) of the Code or the alternative minimum tax; |
|
|
|
|
|
| · | a Non‑U.S. Holder holding our common stock as part of a conversion, constructive sale, wash sale or other integrated transaction or a hedge, straddle or synthetic security; |
|
|
|
|
|
| · | a Non-U.S. Holder that has a “functional currency” other than the U.S. dollar; |
|
|
|
|
|
| · | a Non-U.S. Holder that is a “qualified foreign pension fund” as defined in Section 897(l)(2) of the Code or an entity all of the interests of which are held by one or more “qualified foreign pension funds”; |
|
|
|
|
|
| · | a Non-U.S. Holder that is a corporation that accumulates earnings to avoid U.S. federal income tax; |
|
|
|
|
|
| · | a Non‑U.S. Holder that holds or receives our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; or |
|
|
|
|
|
| · | a Non‑U.S. Holder that at any time owns, directly, indirectly or constructively, 5% or more of our outstanding common stock. |
In addition, this summary does not address any U.S. state or local, or non‑U.S. or other tax consequences, or any U.S. federal income or estate tax consequences for beneficial owners of a Non‑U.S. Holder, including stockholders of a controlled foreign corporation or passive foreign investment company that holds our common stock.
| 82 |
| Table of Contents |
Each Non‑U.S. Holder should consult its own tax advisor regarding the U.S. federal, state, local and non‑U.S. income and other tax consequences of owning and disposing of our common stock.
Distributions on Our Common Stock
We do not currently expect to pay any cash dividends on our common stock. If we make distributions of cash or property (other than certain pro rata distributions of our common stock) with respect to our common stock, any such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax
rules. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a nontaxable return of capital to the extent of the Non‑U.S. Holder’s adjusted tax basis in our common stock and will reduce (but not below zero) such Non‑U.S. Holder’s adjusted tax basis in our common stock. Any remaining excess will be treated as gain from a disposition of our common stock subject to the tax treatment described below in “‑Dispositions of Our common stock.”
Subject to the discussion below on effectively connected income, dividends paid to a Non-U.S. Holder of our common stock will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate provided for by an applicable income tax treaty
, provided the Non-U.S. Holder timely furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may be able to obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Distributions on our common stock that are treated as dividends and that are effectively connected with a Non‑U.S. Holder’s conduct of a trade or business in the United States will be taxed on a net income basis at the regular graduated rates and in the manner applicable to United States persons. An exception may apply if the Non‑U.S. Holder is eligible for, and properly claims, the benefit of an applicable income tax treaty and the dividends are not attributable to a permanent establishment or fixed base maintained by the Non‑U.S. Holder in the United States. In such case, the Non‑U.S. Holder may be eligible for a lower rate under an applicable income tax treaty between the United States and its jurisdiction of tax residence. Dividends that are effectively connected with a Non‑U.S. Holder’s conduct of a trade or business in the United States will not be subject to the U.S. withholding tax if the Non‑U.S. Holder timely provides to the applicable withholding agent a properly executed IRS Form W‑8ECI (or other applicable form) in accordance with the applicable certification and disclosure requirements and certifying that the dividends are effectively connected with the conduct of the Non-U.S. Holder’s trade or business within the United States. A Non‑U.S. Holder treated as a corporation for U.S. federal income tax purposes may also be subject to a “branch profits tax” at a 30% rate (unless the Non‑U.S. Holder is eligible for a lower rate under an applicable income tax treaty) on the Non‑U.S. Holder’s earnings and profits (attributable to dividends on our common stock or otherwise) that are effectively connected with the Non‑U.S. Holder’s conduct of a trade or business within the United States. The amount of taxable earnings and profits is generally reduced by amounts reinvested in the operations of the U.S. trade or business and increased by any decline in its equity.
The certifications described above must be provided to the applicable withholding agent prior to the payment of dividends and must be updated periodically. A Non‑U.S. Holder that does not timely furnish the required documentation, but qualifies for a reduced rate, may be able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS. Non‑U.S. Holders should consult their own tax advisors regarding their eligibility for benefits under any relevant income tax treaty and the manner of claiming such benefits.
The foregoing discussion is subject to the discussions below under “‑Backup Withholding and Information Reporting” and “‑FATCA Withholding.”
| 83 |
| Table of Contents |
Dispositions of Our Common Stock
Subject to the discussions below under “‑Backup Withholding and Information Reporting” and “‑FATCA Withholding,” a Non‑U.S. Holder generally will not be subject to U.S. federal income tax (including U.S. withholding tax) on gain realized on any sale, exchange or other taxable disposition of our common stock unless:
|
| · | the gain is effectively connected with the Non‑U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base maintained by the Non‑U.S. Holder in the United States); in such case, the gain would be subject to U.S. federal income tax on a net income basis at the regular graduated rates and in the manner applicable to United States persons (unless an applicable income tax treaty provides otherwise) and, if the Non‑U.S. Holder is treated as a corporation for U.S. federal income tax purposes, the “branch profits tax” described above may also apply; |
|
|
|
|
|
| · | the Non‑U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of the disposition and meets certain other requirements; in such case, except as otherwise provided by an applicable income tax treaty, the gain, which may be offset by certain U.S. source capital losses, generally will be subject to a flat 30% U.S. federal income tax, even if the Non‑U.S. Holder is not treated as a resident of the United States under the Code; or |
|
|
|
|
|
| · | we are or have been a “United States real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of (i) the five‑year period ending on the date of disposition and (ii) the period that the Non‑U.S. Holder held our common stock. |
Generally, a corporation is a “United States real property holding corporation” if the fair market value of its “United States real property interests” equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. We believe that we are not currently, and we do not anticipate becoming in the future, a United States real property holding corporation. However, because the determination of whether we are a United States real property holding corporation is made from time to time and depends on the relative fair market values of our assets, there can be no assurance in this regard. If we were a United States real property holding corporation, the tax relating to disposition of stock in a United States real property holding corporation generally will not apply to a Non‑U.S. Holder whose holdings, direct, indirect and constructive, constituted 5% or less of our common stock at all times during the applicable period, provided that our common stock are “regularly traded on an established securities market” (as provided in applicable U.S. Treasury regulations) at any time during the calendar year in which the disposition occurs. However, no assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. Non‑U.S. Holders should consult their own tax advisors regarding any possible adverse U.S. federal income tax consequences to them if we are, or were to become, a United States real property holding corporation.
Federal Estate Tax
Any shares of our common stock that are owned (or treated as owned) by an individual who is not a U.S. citizen or resident of the United States (as specially defined for U.S. federal estate tax purposes) at the time of such individual’s death will be included in that individual’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax or other treaty provides otherwise. Such gross estate may be subject to U.S. federal estate tax under the applicable rules.
Backup Withholding and Information Reporting
Backup withholding (currently at a rate of 24%) may apply to dividends paid by U.S. corporations in some circumstances, but will not apply to payments of dividends on our common stock to a Non‑U.S. Holder if the Non‑U.S. Holder provides to the applicable withholding agent a properly executed IRS Form W‑8BEN or W‑8BEN‑E (or other applicable form) certifying under penalties of perjury that the Non‑U.S. Holder is not a United States person or is otherwise entitled to an exemption. However, the applicable withholding agent generally will be required to report to the IRS (and to such Non‑U.S. Holder) payments of dividends on our common stock and the amount of U.S. federal income tax, if any, withheld from those payments. In accordance with applicable treaties or agreements, the IRS may provide copies of such information returns to the tax authorities in the country in which the Non‑U.S. Holder resides.
The gross proceeds from sales or other dispositions of our common stock may be subject, in certain circumstances discussed below, to U.S. backup withholding and information reporting. If a Non‑U.S. Holder sells or otherwise disposes of any of our common stock outside the United States through a non‑U.S. office of a non‑U.S. broker and the disposition proceeds are paid to the Non‑U.S. Holder outside the United States, the U.S. backup withholding and information reporting requirements generally will not apply to that payment. However, U.S. information reporting, but not U.S. backup withholding, will apply to a payment of disposition proceeds, even if that payment is made outside the United States, if a Non‑U.S. Holder sells our common stock through a non‑U.S. office of a broker that is a United States person or has certain enumerated connections with the United States, unless the broker has documentary evidence in its files that the Non‑U.S. Holder is not a United States person and certain other conditions are met or the Non‑U.S. Holder otherwise qualifies for an exemption.
If a Non‑U.S. Holder receives payments of the proceeds of a disposition of our common stock to or through a U.S. office of a broker, the payment will be subject to both U.S. backup withholding and information reporting unless the Non‑U.S. Holder provides to the broker a properly executed IRS Form W‑8BEN or W‑8BEN‑E (or other applicable form) certifying under penalties of perjury that the Non‑U.S. Holder is not a United States person, or the Non‑U.S. Holder otherwise qualifies for an exemption.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be credited against the Non‑U.S. Holder’s U.S. federal income tax liability (which may result in the Non‑U.S. Holder being entitled to a refund), provided that the required information is timely furnished to the IRS.
FATCA Withholding
Sections 1471 through 1474 of the Code and the related Treasury regulations, together with other U.S. Treasury and IRS guidance issued thereunder and intergovernmental agreements, legislation, rules and other official guidance adopted pursuant to such intergovernmental agreements (collectively, “FATCA”) generally impose U.S. federal withholding at a rate of 30% on payments of dividends on our common stock paid to (i) a “foreign financial institution” (as specifically defined in the Code) which does not provide sufficient documentation evidencing either (x) an exemption from FATCA or (y) its compliance (or deemed compliance) with FATCA (which may alternatively be in the form of compliance with an intergovernmental agreement with the United States) in a manner which avoids withholding, or (ii) a “non-financial foreign entity” (as specifically defined in the Code) which does not provide sufficient documentation evidencing either (x) an exemption from FATCA or (y) adequate information regarding certain substantial United States beneficial owners of such entity (if any). An intergovernmental agreement between the United States and an applicable foreign country
may, however, modify these requirements. If FATCA withholding is imposed, a beneficial owner that is not a foreign financial institution generally will be entitled to a refund of any amounts withheld by filing a U.S. federal income tax return containing the required information (which may entail significant administrative burden). Non-U.S. Holders are urged to consult their own tax advisors regarding the effects of FATCA on their investment in our common stock.
While withholding under FATCA would have also applied to payments of gross proceeds from the sale or other disposition of our common stock on or after January 1, 2019 by such applicable non-U.S. entities, U.S. Treasury regulations proposed in December, 2018 eliminate such withholding on payments of gross proceeds entirely. Taxpayers and withholding agents generally may rely on these proposed U.S. Treasury regulations until final U.S. Treasury regulations are issued.
Non‑U.S. Holders are encouraged to consult their tax advisors regarding FATCA.
| 84 |
| Table of Contents |
In connection with this offering, we expect to enter an underwriting agreement with Boustead Securities, LLC (the “representative”) as the representative of the underwriters named below, with respect to the common stock in this offering. Under the terms and subject to the conditions contained in the underwriting agreement, the underwriters will agree to purchase from us on a firm commitment basis the respective number of shares of common stock at the public price less the underwriting discounts and commissions set forth on the cover page of this prospectus, and each of the underwriters has severally agreed to purchase, and we have agreed to sell to the underwriters, at the public offering price per share of common stock less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table.
| Underwriter |
| Number of Shares |
| Boustead Securities, LLC |
|
|
|
|
|
|
| Total |
|
|
The common stock sold by the underwriters to the public will initially be offered at the public offering price set forth on the cover page of this prospectus. Any common stock sold by the underwriters to securities dealers may be sold at a discount from the public offering price not to exceed $ per share. If all of the shares are not sold at the initial offering price, the representative may change the offering price and the other selling terms. The representative has advised us that the underwriters do not intend to make sales to discretionary accounts. The underwriting agreement will provide that the obligations of the underwriters to pay for and accept delivery of the shares are subject to the passing upon certain legal matters by counsel and certain conditions such as confirmation of the accuracy of representations and warranties by us about our financial condition and operations and other matters.
Over‑Allotment Option
We have granted the representative an option to purchase up to an additional [*] shares of common stock, representing 15% of the aggregate shares of common stock sold in this offering from us at the public offering price, less underwriting discounts and commissions, within 30 days from the date of this prospectus to cover over-allotments, if any. This offering is being conducted on a firm commitment basis. Any shares of common stock issued or sold under the option will be issued and sold on the same terms and conditions as the other shares that are the subject of this offering.
Discounts and Commissions; Expenses
The underwriting discounts and commissions are a cash fee equal to 7.0% of gross proceeds from the sale of common stock in this offering. We have been advised by the representative that the underwriters propose to offer the common stock to the public at the public offering price set forth on the cover of this prospectus and to dealers at a price that represents a concession not in excess of $ per share under the public offering price. After the offering, the representative may change the public offering price and other selling terms.
| 85 |
| Table of Contents |
The following table summarizes the public offering price and the underwriting discounts and commissions payable to the underwriters by us in connection with this offering (assuming a public offering price of $ per share):
|
|
| Per Share |
|
| Without Over-Allotment Option |
|
| With Over-Allotment Option |
|
|||
| Initial public offering price |
| $ |
|
|
| $ |
|
|
| $ |
|
|
| Underwriting discounts and commissions (7.0%) |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-accountable expense allowance (1.0%) |
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds to us, before expenses |
| $ |
|
|
| $ |
|
|
| $ |
|
|
We have agreed to pay the representative a non-accountable expense allowance equal to 1.00% of the gross proceeds received at the closing of this offering.
We have agreed to reimburse the representative for reasonable out-of-pocket expenses incurred by it in connection with this offering, regardless of whether the offering is consummated, up to $50,000. Any advance payments will be returned to us to the extent any portion of the advance is not actually incurred, in accordance with FINRA Rule 5110(g)(4)(A).
We estimate that our total expenses of the offering, excluding the estimated underwriting discounts and commissions and the non-accountable expense allowance, will be approximately $ .
Underwriter’s
We have also agreed to issue to the underwriter a warrant to purchase a number of our securities equal to 7.0% of the common stock sold in this offering. The underwriters’ warrant will have an exercise price equal to 100% of the public offering price shares set forth on the cover of this prospectus (or $[*] per share) and may be exercised on a cashless basis. The underwriters’ warrant is not redeemable by us. This prospectus also covers the sale of the underwriter’s warrant and the shares of common stock issuable upon the exercise of the underwriter’s warrant.
The underwriter’s warrant and the underlying securities have been deemed compensation by FINRA, and are therefore subject to lock-up pursuant to FINRA Rule 5110(e)(1). In accordance with FINRA Rule 5110(e)(1), neither the warrant nor any of the underlying securities issued upon exercise of the warrant may be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of such securities by any person, for a period of 180 days immediately following the commencement of sales of this offering subject to certain exceptions permitted by FINRA Rule 5110(e)(2).
Indemnification
We have agreed to indemnify the representative and the other underwriters, if any, against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to payments that the representative and the other underwriters may be required to make for these liabilities.
Lock‑Up Agreements
Our executive officers, directors following this offering and other security holder(s) of five percent (5%) or more have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any shares of our common stock for a period of six months following the closing of this offering, subject to certain exceptions.
Notwithstanding the above, the representative of this offering may engage in stabilization activities as described below. The representative may in its sole discretion and at any time without notice release some or all of the shares subject to lock‑up agreements prior to the expiration of the lock‑up period. When determining whether or not to release shares from the lock‑up agreements, the representative will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
| 86 |
| Table of Contents |
We will not, without the prior written consent of the representative, from the date of execution of the Underwriting Agreement and continuing for a period of three months from such date (the “Lock-Up Period”), (a) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of our capital stock; (b) file or caused to be filed any registration statement with the Commission relating to the offering of any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of our capital stock; (c) complete any offering of our debt securities, other than entering into a line of credit with a traditional bank or (d) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our capital stock, whether any such transaction described in clause (a), (b), (c) or (d) above is to be settled by delivery of shares of our capital stock or such other securities, in cash or otherwise.
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the underwriters may engage in stabilizing transactions, over‑allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act:
|
| · | Stabilizing transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum. |
|
|
|
|
|
| · | Over‑allotment transactions involve sales by the underwriters of securities in excess of the number of securities the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of securities over‑allotted by the underwriters is not greater than the number of securities that they may purchase in the over‑allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over‑allotment option. The underwriters may close out any covered short position by either exercising its over‑allotment option and/or purchasing securities in the open market. |
|
|
|
|
|
| · | Syndicate covering transactions involve purchases of the securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared to the price at which they may purchase securities through the over‑allotment option. A naked short position occurs if the underwriters sells more securities than could be covered by the over‑allotment option. This position can only be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in this offering. |
|
|
|
|
|
| · | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when securities originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of the securities. As a result, the price of our shares of common stock may be higher than the price that might otherwise exist in the open market. These transactions may be discontinued at any time.
| 87 |
| Table of Contents |
Determination of Offering Price
In determining the public offering price, we and the representative have considered a number of factors, including:
|
| · | the information set forth in this prospectus and otherwise available to the representative; |
|
|
|
|
|
| · | our prospects and the history and prospects for the industry in which we compete; |
|
|
|
|
|
| · | an assessment of our management; |
|
|
|
|
|
| · | our prospects for future revenues and earnings; |
|
|
|
|
|
| · | the general condition of the securities markets at the time of this offering; |
|
|
|
|
|
| · | the recent market prices of, and demand for, publicly traded securities of generally comparable companies, as well as the recent market price of our common stock; and |
|
|
|
|
|
| · | other factors deemed relevant by the representative and us. |
The estimated public offering price set forth on the cover page of this preliminary prospectus is subject to change as a result of market conditions and other factors.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be delivered to potential investors by the underwriters in this offering. In addition, the common stock may be sold by the underwriters to securities dealers who resell to online brokerage account holders. The prospectus in electronic format will be identical to the paper version of such prospectus. Other than the prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the representative in its capacity as representative and should not be relied upon by investors.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Other
Peter Conley has served as our Chief Financial Officer and SVP Intellectual Property since May 2022. Mr. Conley has served as Senior Managing Director and Head of Intellectual Property Banking at Boustead Securities, LLC since October 2014. Since joining the Company, Mr. Conley has not been active at Boustead Securities, LLC.
Proskauer Rose LLP, Los Angeles, CA has acted as our counsel in connection with the preparation of this prospectus and the registration statement of which this prospectus is a part. Brownstein Hyatt Farber Schreck, LLP, Las Vegas, NV will pass upon the validity of the shares of common stock being registered by the registration statement of which this prospectus is a part. ArentFox Schiff LLP, Washington, DC is acting as counsel to the underwriters.
The consolidated financial statements of Know Labs, Incorporated and subsidiaries as of September 30, 2022 and 2021 and for the two years then ended, have been incorporated in this prospectus by reference to the Annual Report on Form 10-K for the year ended September 30, 2022, in reliance upon the report of BMP LLP, an independent registered public accounting firm, given on the authority of said firm as experts in accounting and auditing.
| 88 |
| Table of Contents |
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The following documents filed with the SEC are incorporated by reference into this prospectus:
|
| · | our Annual Report on Form 10-K for the year ended September 30, 2022, filed on December 20, 2022; |
|
|
|
|
|
| · | our Quarterly Reports on Form 10-Q for the periods ended December 31, 2022, March 31, 2023, and June 30, 2023, filed February 14, 2023, May 15, 2023, and August 14, 2023, respectively; |
|
|
|
|
|
| · | our Current Reports on Form 8-K, filed on December 9, 2022, December 9, 2023, January 4, 2023, January 23, 2023, January 25, 2023, January 25, 2023, January 27, 2023, August 14, 2023 and August 18, 2023 (other than any portions thereof deemed furnished and not filed); |
|
|
|
|
|
| · | our Definitive Proxy Statement on Schedule 14A filed with the SEC on August 4, 2023; and |
|
|
|
|
|
| · | the description of our common stock contained in our Registration Statement on Form 8-A, filed with the SEC on September 15, 2022, including any amendments thereto or reports filed for the purposes of updating this description, including Exhibit 4.5 to our Annual Report on Form 10-K for the year ended September 30, 2022, filed with the SEC on December 20, 2022. |
We also incorporate by reference all documents we file pursuant to Section 13(a), 13(c), 14 or 15 of the Exchange Act (other than any portions of filings that are furnished rather than filed pursuant to Items 2.02 and 7.01 of a Current Report on Form 8-K) after the date of the initial registration statement of which this prospectus is a part and prior to effectiveness of such registration statement. All documents we file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the termination of the offering are also incorporated by reference and are an important part of this prospectus.
Any statement contained in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for the purposes of this registration statement to the extent that a statement contained herein or in any other subsequently filed document which also is or deemed to be incorporated by reference herein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this registration statement.
You may request and obtain a copy of any of the filings incorporated herein by reference, at no cost, by writing or telephoning us at the following address or phone number:
Know Labs Inc.
500 Union Street, Suite 810
Seattle, WA 98101
(206) 903-1351
ask@knowlabs.co
www.knowlabs.co.
| 89 |
| Table of Contents |
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement, of which this prospectus is a part, on Form S‑1 with the SEC relating to this offering. This prospectus does not contain all of the information in the registration statement and the exhibits included with the registration statement. For further information pertaining to us and the common stock to be sold in this offering, you should refer to the registration statement and its exhibits. References in this prospectus to any of our contracts, agreements or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contracts, agreements or documents. You can read our SEC filings, including the registration statement, on the internet at the SEC’s website. The address of that site is http://www. sec. gov.
We are subject to the informational requirements of the Exchange Act, and, in accordance with the Exchange Act, we file reports, proxy and information statements and other information with the SEC. Such annual, quarterly and special reports, proxy and information statements and other information can be inspected and copied at the locations set forth above. We also make these documents publicly available, free of charge, on our website at www.knowlabs.co. as soon as reasonably practicable after filing such documents with the SEC. Information on, or accessible through, our website is not part of this prospectus. You may also request a copy of these filings, at no cost, by writing us at 500 Union Street, Suite 810, Seattle WA 98101, or ask@knowlabs.co, or telephoning us at (206) 903-1351.
| 90 |
| Table of Contents |

Know Labs, Inc.
Shares of Common Stock
______________________
PRELIMINARY PROSPECTUS
______________________
Sole Book-Running Manager
BOUSTEAD SECURITIES, LLC
, 2023
Until , 2023, 25 days after the date of this prospectus, all dealers that buy, sell or trade our securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale of common shares being registered. All amounts, other than the SEC registration fee, NYSE American listing fee and FINRA filing fee, are estimates. We will pay all these expenses.
| Securities and Exchange Commission registration fee |
| $ | 1,356.17 |
|
|
|
| FINRA filing fee |
|
|
|
|
| * |
| Accountant’s fees and expenses |
|
|
|
|
| * |
| Legal fees and expenses |
|
|
|
|
| * |
| Blue Sky fees and expenses |
|
|
|
|
| * |
| Transfer agent’s fees and expenses |
|
|
|
|
| * |
| Printing and related fees |
|
|
|
|
| * |
| Miscellaneous |
|
|
|
|
| * |
|
|
|
|
|
|
|
|
| Total expenses |
| $ |
|
|
| * |
* To be provided by amendment.
Item 14. Indemnification of Directors and Officers
We are a Nevada corporation. The Nevada Revised Statutes, or NRS, and certain provisions of our articles of incorporation and bylaws under certain circumstances provide for indemnification of our directors, officers, employees and agents against certain liabilities which they may incur in such capacities. A summary of the circumstances in which such indemnification is set forth below, but this description is qualified in its entirety by reference to our articles of incorporation and bylaws and to the relevant statutory provisions, including NRS 78.7502, 78.751 and 78.752.
In general, our articles of incorporation provide that any officer, director, employee or agent may be indemnified against expenses, fines, settlements or judgments arising in connection with a legal proceeding to which such person is a party, if that person acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the Company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful. Our bylaws further provide that each person who was or is made a party or is threatened to be made a party to any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact such person is or was a director or officer of the Company or is or was serving at the request of the Company as a director or officer of another enterprise, shall be indemnified and held harmless by the Company to the fullest extent permitted by the NRS against all expense, liability and loss (including attorneys’ fees, judgments, fines or penalties and amounts paid in settlement) reasonably incurred or suffered by such person in connection therewith.
NRS 78.751 provides that indemnification may not be made to or on behalf of any director or officer finally adjudged by a court of competent jurisdiction, after exhaustion of any appeals taken therefrom, to be liable for intentional misconduct, fraud or a knowing violation of the law if such intentional misconduct, fraud or a knowing violation of the law was material to the cause of action. However, NRS 78.752 permits us to purchase and maintain insurance on behalf of our directors, officers, employees or agents against any liability asserted against or incurred by such person in any such capacity or arising out of such person’s status as such, whether or not we would have the power to indemnify such person against such liabilities.
To the maximum extent permitted by law, our articles of incorporation eliminate or limit the liability of our directors to us or our stockholders for monetary damages for breach of a director’s fiduciary duty as a director. NRS 78.138(7) further provides that, subject to limited statutory exceptions and unless the articles of incorporation or an amendment thereto (in each case filed on or after October 1, 2003) provide for greater individual liability, a director or officer is not individually liable to a Nevada corporation or its stockholders or creditors for any damages as a result of any act or failure to act in his or her capacity as a director or officer unless the presumption established by NRS 78.138(3) has been rebutted and it is proven that (i) his or her act or failure to act constituted a breach of his or her fiduciary duties as a director or officer, and (ii) such breach involved intentional misconduct, fraud or a knowing violation of the law.
| II-1 |
We have entered into separate indemnification agreements with our directors and executive officers. Each indemnification agreement provides, among other things, for indemnification to the fullest extent permitted by law and our articles of incorporation and bylaws against any and all expenses, judgments, fines, penalties and amounts paid in settlement of any claim. The indemnification agreements will provide for the advancement or payment of all expenses to the indemnitee and for reimbursement to us if it is found that such indemnitee is not entitled to such indemnification under applicable law and our articles of incorporation and bylaws.
We have a directors’ and officers’ liability insurance policy in place pursuant to which its directors and officers are insured against certain liabilities, including certain liabilities under the Securities Act and the Exchange Act of 1934, as amended.
The underwriting agreement, filed as Exhibit 1.1 to this registration statement, will provide for indemnification, under certain circumstances, by the underwriter of us and our officers and directors for certain liabilities arising under the Securities Act or otherwise.
As far as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us under the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 15. Recent Sales of Unregistered Securities
During the past three years, we issued the following securities, which were not registered under the Securities Act.
All of the offerings and sales described below were deemed to be exempt under Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act. No advertising or general solicitation was employed in offering the securities, the offerings and sales were made to a limited number of persons, all of whom were accredited investors and transfer was restricted by the company in accordance with the requirements of Regulation D and the Securities Act. All issuances to accredited and non‑accredited investors were structured to comply with the requirements of the safe harbor afforded by Rule 506 of Regulation D, including limiting the number of non‑accredited investors to no more than 35 investors who have sufficient knowledge and experience in financial and business matters to make them capable of evaluating the merits and risks of an investment in our securities. We have not employed any underwriters in connection with any of the below transactions, and, except as otherwise noted below, the individuals and entities to whom we issued securities are not affiliated with us. Except as noted below, none of the holders of the securities have any contractual rights to have such securities registered with the Securities and Exchange Commission.
Year Ended September 30, 2021
We issued 3,676,542 shares of common stock at an average price of $0.582 per share related to the exercise of warrants.
We issued 97,000 shares related to services. The shares were valued at the fair market value of $202,820.
We issued 16,875 shares related to the exercise of stock option grants at $1.38 per share.
We issued 480,600 shares of common stock at an average price of $2.00 per share or $961,200 related to the conversion of Particle Simple Agreements for Future Equity into shares of the Company’s common stock.
Year Ended September 30, 2022
We issued 1,045,724 shares of common stock related to warrant exercises and received $838,487.
We issued 26,293 shares related to the exercise of stock option grants and received $26,887.
The Company issued 104,634 shares each to three directors and three consultants at $1.749 per share.
| II-2 |
Ten Months Ended August 31, 2023
We issued 50,000 shares of common stock related to the exercise of warrants and received $12,500. .
We issued 2,632,727 shares of common stock related to warrant exercises and received $374,835.
On June 27, 2023, Mr. Struve converted dividends of $350,696 into 1,402,784 shares of its common stock related to the conversion of Series D preferred stock.
Item 16. Exhibits.
(a) Exhibits.
| Exhibit No. |
| Description |
|
|
|
|
| 1.1** |
| Form of Underwriting Agreement |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
| 4.2** |
| Form of Underwriter Warrant |
| 5.1** |
| Opinion of Brownstein Hyatt Farber Schreck, LLP |
| 5.2** |
| Opinion of Proskauer Rose LLP |
|
| ||
|
| ||
|
|
| II-3 |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| II-4 |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| Consent of BPM LLP, Independent Registered Public Accounting Firm |
|
| 23.2** |
| Consent of Brownstein Hyatt Farber Schreck, LLP (included in Exhibit 5.1) |
| 23.3** |
| Consent of Proskauer Rose LLP (included in Exhibit 5.2) |
| 24.1* |
| Power of Attorney (included in the signature page) |
|
|
________________
| † | Executive compensation plan or arrangement. |
| * | Filed herewith. |
| ** | To be filed by amendment. |
(b) Financial Statement Schedules.
All financial statement schedules are omitted because the information called for is not required or is shown either in the financial statements or in the notes thereto.
Item 17. Undertakings
The undersigned Registrant hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
|
| i. | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
|
|
|
|
|
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
|
|
|
|
|
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| II-5 |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
|
|
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
|
|
|
| (4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
|
| A. | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
|
|
|
|
|
| B. | Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| (5) | That for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
|
| i. | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
|
|
|
|
|
| ii. | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
|
|
|
|
|
| iii. | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
|
|
|
|
|
| iv. | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| II-6 |
| (6) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
|
|
|
| (7) | The undersigned registrant hereby undertakes that: |
|
| i. | For purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; |
|
|
|
|
|
| ii. | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(I) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and |
|
|
|
|
|
| iii. | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| II-7 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Seattle, State of Washington, on September 5, 2023.
| Know Labs, Inc.
|
|
||
|
| By: | /s/ Ronald P. Erickson |
|
|
|
| Ronald P. Erickson Chief Executive Officer and Director |
|
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints each of Ronald P. Erickson and Peter Conley as his or her true and lawful attorneys‑in‑fact and agents with full power of substitution and resubstitution, for him and his name, place and stead, in any and all capacities, to sign any or all amendments (including post‑effective amendments) to this registration statement and to file a new registration statement under Rule 461, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys‑in‑fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the foregoing, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys‑in‑fact and agents, or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| SIGNATURE |
| TITLE |
| DATE |
|
|
|
|
|
|
| /s/ Ronald P. Erickson |
| Chief Executive Officer and Director (Principal Executive Officer) |
| September 5, 2023 |
| Ronald P. Erickson |
|
|
|
|
|
|
|
|
|
|
| /s/ Peter Conley |
| Chief Financial Officer (Principal Financial Officer) |
| September 5, 2023 |
| Peter Conley |
|
|
|
|
|
|
|
|
|
|
| /s/ Jon Pepper |
| Director |
| September 5, 2023 |
| Jon Pepper |
|
|
|
|
|
|
|
|
|
|
| /s/ William A. Owens |
| Director |
| September 5, 2023 |
| William A. Owens |
|
|
|
|
|
|
|
|
|
|
| /s/ Ichiro Takesako |
| Director |
| September 5, 2023 |
| Ichiro Takesako |
|
|
|
|
| II-8 |