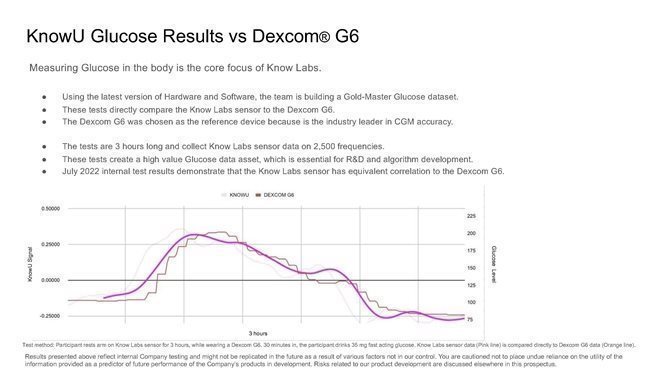
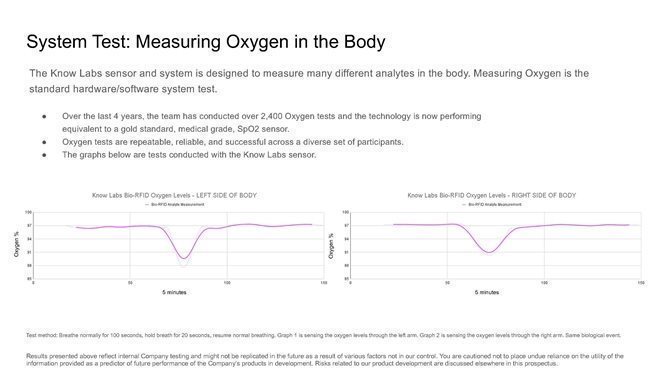
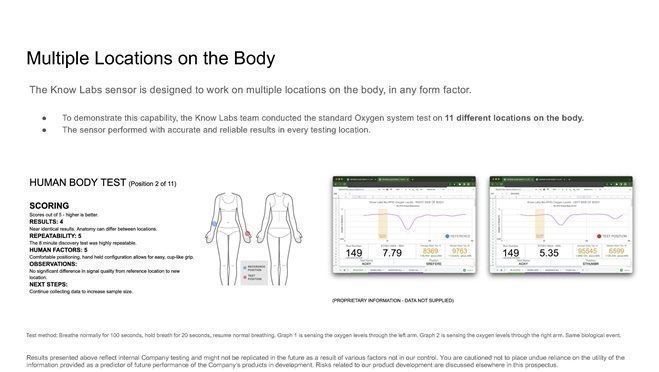
424B4: Prospectus filed pursuant to Rule 424(b)(4)
Published on September 19, 2022
Filed Pursuant to Rule 424(b)(4)
Registration No. 333-266423
PROSPECTUS

Know Labs, Inc.
3,600,000 Shares of Common Stock
This is a firm commitment public offering. We are offering 3,600,000 shares of our common stock, par value $0.001 per share, at a public offering price of $2.00 per share.
Prior to this offering, our common stock was traded on the OTC Market Group Inc.’s QB tier, or OTCQB, under the symbol “KNWN.” In connection with this offering, we received approval from NYSE American for the listing of our common stock under the symbol “KNW.” Our common stock will commence trading on September 16, 2022.
Investing in our common stock involves a high degree of risk. See the section of this prospectus entitled “Risk Factors” beginning on page 7 for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
| Per Share |
|
| Total |
|
||
| Public offering price |
| $ | 2.00 |
|
| $ | 7,200,000 |
|
| Underwriting discounts and commissions(1) |
| $ | 0.14 |
|
| $ | 504,000 |
|
| Proceeds, before expenses, to us(2) |
| $ | 1.86 |
|
| $ | 6,696,000 |
|
|
| (1) | Does not include a non-accountable expense allowance equal to 1.00% of the gross proceeds of this offering payable to Boustead Securities, LLC, the representative of the underwriters. We have also agreed to issue warrants to the representative of the underwriters. See “Underwriting” for a complete description of the compensation arrangements. |
|
|
|
|
|
| (2) | Excludes fees and expenses payable by us. The total amount of our expenses related to this offering is set forth in the section entitled “Underwriting.” |
We have granted a 45-day option to the underwriters to purchase up to 540,000 additional shares of our common stock, solely to cover over-allotments, if any, at the public offering price less the underwriting discounts.
The underwriters are offering the shares for sale on a firm commitment basis. The underwriters expect to deliver the shares to the purchasers on or about September 20, 2022.
BOUSTEAD SECURITIES, LLC
Prospectus dated September 15, 2022

| ii |
| iii |
|
|
| Page |
|
|
|
|
|
|
|
| 3 |
|
|
|
| 9 |
|
|
|
| 21 |
|
|
|
| 22 |
|
|
|
| 23 |
|
|
|
| 24 |
|
|
|
| 25 |
|
|
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
| 26 |
|
|
| 34 |
|
|
|
| 42 |
|
|
|
| 47 |
|
|
|
| 53 |
|
|
|
| 56 |
|
|
|
| 57 |
|
|
|
| 64 |
|
|
| Material U.S. Federal Tax Considerations For Non-U.S. Holders of Our Common Stock |
| 65 |
|
|
| 68 |
|
|
|
| 72 |
|
|
|
| 72 |
|
|
|
| 72 |
|
|
|
| F-1 |
|
This prospectus constitutes a part of a registration statement on Form S-1 (or, together with all amendments and exhibits thereto, the Registration Statement) filed by us with the Securities and Exchange Commission, or the SEC, under the Securities Act of 1933, as amended, or the Securities Act. As permitted by the rules and regulations of the SEC, this prospectus omits certain information contained in the Registration Statement, and reference is made to the Registration Statement and related exhibits for further information with respect to Know Labs, Inc. and the securities offered hereby. With regard to any statements contained herein concerning the provisions of any document filed as an exhibit to the Registration Statement or otherwise filed with the SEC, in each instance reference is made to the copy of such document so filed. Each such statement is qualified in its entirety by such reference.
You should rely only on the information contained in this prospectus or in any related free-writing prospectus. We and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectus prepared by us or on our behalf or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any information that others may give you.
This prospectus is an offer to sell only the common stock offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. We are not making an offer to sell these shares of common stock in any jurisdiction where the offer or sale is not permitted or where the person making the offer or sale is not qualified to do so or to any person to whom it is not permitted to make such offer or sale. The information contained in this prospectus is current only as of the date of the front cover of the prospectus. Our business, financial condition, operating results and prospects may have changed since that date.
Persons who come into possession of this prospectus and any applicable free writing prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus and any such free writing prospectus applicable to that jurisdiction. See “Underwriting” for additional information on these restrictions.
Until and including October 10, 2022 (the 25th day after the date of this prospectus), all dealers effecting transactions in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
| 1 |
| Table of Contents |
For investors outside of the United States: Neither we nor the underwriters have taken any action to permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
For purposes of this Registration Statement, “Company,” “we” or “our” refers to Know Labs, Inc. and its subsidiaries, unless otherwise required by the context.
INDUSTRY AND MARKET DATA
This prospectus includes statistical, market and industry data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we are responsible for all of the disclosures contained in this prospectus, including such statistical, market and industry data, we have not independently verified any of the data from third-party sources, nor have we ascertained the underlying economic assumptions relied upon therein. In addition, while we believe the market opportunity information included in this prospectus is generally reliable and is based on reasonable assumptions, such data involves risks and uncertainties, including those discussed under the heading “Risk Factors.”
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
We own or have rights to trademarks, service marks and trade names that we use in connection with the operation of our business, including our corporate name, logos and website names. Other trademarks, service marks and trade names appearing in this prospectus are the property of their respective owners. Solely for convenience, some of the trademarks, service marks and trade names referred to in this prospectus are listed without the® and ™ symbols, but we will assert, to the fullest extent under applicable law, our rights to our trademarks, service marks and trade names.
This prospectus includes trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included in this prospectus are the property of their respective owners.
| 2 |
| Table of Contents |
|
This summary highlights selected information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our securities. You should carefully read the entire prospectus, including the risks associated with an investment in our company discussed in the “Risk Factors” section of this prospectus, before making an investment decision. Some of the statements in this prospectus are forward-looking statements. See the section titled “Cautionary Statement Regarding Forward-Looking Statements.”
OUR COMPANY
Overview
Know Labs is focused on the development and commercialization of proprietary biosensor technologies which, when paired with our artificial intelligence, or AI, deep learning platform, are capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify and measure the unique “signature” of said materials or analytes. We call these our “Bio-RFID™” technology platform when pertaining to radio and microwave spectroscopy; and “ChromaID” technology platform when pertaining to optical spectroscopy. The data obtained with our biosensor technology is analyzed with our trade secret algorithms which are driven by our AI deep learning platform. There are a significant number of analytes in the human body that relate to health and wellness. Our focus is upon those analytes relating to human health, the identification of which provide diagnostic information and require, by their nature, clearance by the United States Food and Drug Administration.
Our Opportunity
The first applications of our Bio-RFID technology will be in a product marketed as a non-invasive glucose monitor. It will provide the user with real time information on their blood glucose levels. This product will require US Food and Drug Administration, or FDA, clearance prior to its introduction to the market, which we plan to pursue. The addressable market for a non-invasive blood glucose monitor is very large and includes, globally, not only the approximately 450 million individuals suffering from diabetes but the more than one billion individuals with pre-diabetes.
Our Products and Services
We are currently undertaking internal development work on potential products for the commercial marketplace. We have announced the development of our non-invasive glucose monitor and our desire to obtain FDA clearance for the marketing of this product. We have also announced the engagement of a manufacturing partner we will work with to bring this product to market. We will make further announcements regarding our products as development, testing, manufacturing, and regulatory approval work progresses.
Our Competitive Strengths
We believe our key competitive strengths include:
|
||
|
|
|
|
|
| · | Bio-RFID’s ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so concurrently, and in real time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring. |
|
|
|
|
|
| · | Non-Invasive: Our Bio-RFID technology is non-invasive, using radio waves to identify and measure what is going on inside the body. |
|
|
|
|
|
| · | Form Factor Agnostic: Our Bio-RFID technology platform that can be integrated into a variety of wearable, mobile or bench-top form factors. |
|
|
|
|
|
| · | Pain-free: No needles nor invasive transmitters in your body, making Bio-RFID sensors convenient and pain-free. |
|
|
|
|
|
| · | No Consumables: Expensive supplies, such as test strips and lancets, are not required to operate Bio-RFID devices. |
|
|
|
|
| 3 |
| Table of Contents |
|
Our Growth Strategies
The key elements of our strategy to grow our business include: |
||
|
|
|
|
|
| · | Initially, entering the diabetes continuous glucose monitoring, or CGM, market with our non-invasive CGM product. |
|
|
|
|
|
| · | Following our entry into the CGM market, entering other clinical monitoring markets for continuous, non-invasive hormone, medication metabolite, endocrinology components and biomolecular monitoring. |
|
|
|
|
|
| · | Applying our Bio-RFID platform technology to lifestyle analysis, clinical trials and chronic illnesses. |
|
|
|
|
| We believe that potential use cases include real time wearable medication monitoring and detection of, for example, ovulation and hormone deficiency.
Impact of COVID-19 Pandemic
Over the past two years the impact of COVID-19 has had adverse effects on our business by slowing down our ability to work with third parties outside of Seattle on testing and validation. We have witnessed supply chain related delays and increasing costs due to pandemic relate inflation. It is difficult to predict what other adverse effects, if any, COVID-19 and related matters can have on our business, or against the various aspects of same.
It is difficult to isolate the impact of the pandemic on our business, results of operations, financial condition and our future strategic plans.
We may experience long-term disruptions to our operations resulting from changes in government policy or guidance; quarantines of employees, customers and suppliers in areas affected by the pandemic and the presence of new variants of COVID-19; and closures of businesses or manufacturing facilities critical to its business or supply chains. We are actively monitoring, and will continue to actively monitor, the pandemic and the potential impact on its operations, financial condition, liquidity, suppliers, industry and workforce.
For a further discussion of the impact of the COVID-19 pandemic on our business, please see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Impact of COVID-19 Pandemic”.
Corporate Information
We were incorporated under the laws of the State of Nevada on October 8, 1998. Our executive offices are located at 500 Union Street, Suite 810, Seattle, WA 98101. Our telephone number is (206) 903-1351 and our principal website address is located at www.knowlabs.co. The information on our website is not incorporated by reference in and is not deemed a part of this prospectus.
|
||
| 4 |
| Table of Contents |
|
THE OFFERING |
||
|
|
|
|
| Common stock offered by us:
|
| 3,600,000 shares of common stock (or 4,140,000 shares of common stock if the underwriters exercise the over-allotment option in full).
|
| Offering price:
|
| $2.00 per share.
|
| Common stock outstanding immediately before the offering:
|
| 43,849,281 shares of common stock.
|
| Common stock outstanding immediately after the offering:
|
| 47,449,281 shares of common stock (or 47,989,281 shares if the underwriters exercise the over-allotment option in full).
|
| Underwriting; Over-allotment option:
|
| This offering is being conducted on a firm commitment basis. The underwriters are obligated to take and pay for all of the shares of common stock if any such shares are taken. We have granted to the underwriters an option for a period of 45 days from the date of this prospectus to purchase up to 540,000 additional shares (constituting 15% of the total number of shares of common stock to be offered in this offering) of common stock from us at the public offering price, less the underwriting discounts and commissions, to cover over-allotments, if any.
|
| Representative’s warrants:
|
| We have agreed to issue to Boustead Securities, LLC, the representative of the underwriters (or its permitted assignees), warrants to purchase up to a total of 252,000 shares of common stock, or 289,800 shares if the underwriters exercise the over-allotment option in full (equal to 7% of the aggregate number of the shares sold in this offering), at an exercise price of $2.40 (equal to 120% of the public offering price of the common stock sold in this offering). The representative’s warrants will be exercisable at any time, and from time to time, in whole or in part, commencing from the closing of the offering and expiring five (5) years from commencement of sales in the offering and will have a cashless exercise provision. See “Underwriting” for more information.
|
| Use of proceeds:
|
| We estimate that the net proceeds from this offering will be approximately $6.35 million, or approximately $7.37 million if the underwriters exercise the option to purchase additional shares to cover over-allotments, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the proceeds from this offering for research and development, sales and marketing, general and administrative, capital investments and working capital. See “Use of Proceeds.”
|
| Risk factors:
|
| Investing in our securities involves a high degree of risk. As an investor, you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section beginning on page 7 before deciding to invest in our common stock.
|
| Lock-up
|
| Our executive officers and directors have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any shares of our common stock for a lock-up period of six months following the closing of this offering, subject to certain exceptions. See “Underwriting” for more information.
|
| Proposed trading market and symbol
|
| Prior to this offering, our common stock was traded on the OTCQB under the symbol “KNWN.” In connection with this offering, we received approval from NYSE American for the listing of our common stock under the symbol “KNW.” Our common stock will commence trading on September 16, 2022.
|
| 5 |
| Table of Contents |
|
The number of shares of common stock outstanding immediately following this offering is based on 43,849,281 shares outstanding as of the date of this prospectus and excludes: |
||
|
|
|
|
|
| · | 21,067,370 shares of our common stock issuable upon the exercise of options which we granted to our officers, directors, and employees under the 2021 Plan (as defined below) at a weighted average exercise price of $1.632 per share (includes 13,966,370 shares of common stock issuable upon the exercise of options originally granted under our 2011 Plan (as defined below) at a weighted average exercise price of $1.483 per share, and now subsumed under our 2021 Plan); |
|
|
|
|
|
| · | 15,582,750 additional shares of our common stock that are reserved for issuance under the 2021 Plan; |
|
|
|
|
|
| · | 8,108,356 shares of our common stock issuable upon the conversion of our series C convertible preferred stock and series D convertible preferred stock; |
|
|
|
|
|
| · | 9,020,264 shares of our common stock issuable upon the conversion of convertible debentures; |
|
|
|
|
|
| · | 21,644,013 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $1.004 per share; and |
|
|
|
|
|
| · | up to 289,800 shares of our common stock issuable upon exercise of the representative’s warrants issued in connection with this offering. |
|
|
|
|
| 6 |
| Table of Contents |
|
SUMMARY FINANCIAL INFORMATION
The following tables summarize certain financial data regarding our business and should be read in conjunction with our financial statements and related notes contained elsewhere in this prospectus and the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Our summary financial data as of September 30, 2021 and 2020 are derived from our audited financial statements included elsewhere in this prospectus. Our summary financial data as of June 30, 2022 and 2021 are derived from our unaudited financial statements included elsewhere in this prospectus. All financial statements included in this prospectus are prepared and presented in accordance with generally accepted accounting principles in the United States, or GAAP. The summary financial information is only a summary and should be read in conjunction with the historical financial statements and related notes contained elsewhere herein. The financial statements contained elsewhere fully represent our financial condition and operations; however, they are not indicative of our future performance. |
||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||
|
| Nine Months Ended June 30, |
|
| Years Ended September 30, |
|
|||||||||||
| Statements of Operations Data |
| 2022 |
|
| 2021 |
|
| 2021 |
| 2020 |
|
|||||
|
| (unaudited) |
|
| (unaudited) |
|
|
|
|
||||||||
| Revenue |
| $ | 4,360,087 |
|
| $ | - |
|
| $ | - |
|
| $ | 121,939 |
|
| Cost of sales |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 69,726 |
|
| Gross profit |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 52,213 |
|
| Operating expenses |
|
| 11,097,948 |
|
|
| 8,028,208 |
|
|
| 10,446,148 |
|
|
| 6,878,141 |
|
| Operating loss |
|
| (6,737,861 | ) |
|
| (8,028,208 | ) |
|
| (10,446,148 | ) |
|
| (6,825,928 | ) |
| Other income and (expense) |
|
| (7,762,782 | ) |
|
| (9,735,604 | ) |
|
| (14,914,065 | ) |
|
| (6,736,713 | ) |
| Net loss |
| $ | (14,500,643 | ) |
| $ | (17,763,812 | ) |
| $ | (25,360,213 | ) |
| $ | (13,562,641 | ) |
| Basic and diluted loss per share |
| $ | (0.37 | ) |
| $ | (0.64 | ) |
| $ | (0.86 | ) |
| $ | (0.62 | ) |
| Weighted average shares of common stock outstanding- basic and diluted |
|
| 39,032,860 |
|
|
| 27,651,679 |
|
|
| 29,370,596 |
|
|
| 21,791,058 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As of June 30, |
|
| As of September 30, |
|
||||||
|
|
| 2022 |
|
| 2021 |
|
| 2020 |
|
|||
| Balance Sheet Data |
| (unaudited) |
|
|
|
|
|
|
||||
| Cash and cash equivalents |
| $ | 8,351,945 |
|
| $ | 12,258,218 |
|
| $ | 4,298,179 |
|
| Total current assets |
|
| 8,398,091 |
|
|
| 12,258,218 |
|
|
| 4,298,179 |
|
| Total assets |
|
| 9,627,478 |
|
|
| 12,889,491 |
|
|
| 4,682,147 |
|
| Total current liabilities |
|
| 4,610,949 |
|
|
| 11,469,158 |
|
|
| 6,347,632 |
|
| Total liabilities |
|
| 4,708,210 |
|
|
| 11,647,328 |
|
|
| 6,597,058 |
|
| Total stockholder’s equity (deficit) |
|
| 4,919,268 |
|
|
| 1,242,163 |
|
|
| (1,914,911 | ) |
| Total liabilities and stockholder’s equity |
| $ | 9,627,478 |
|
| $ | 12,889,491 |
|
| $ | 4,682,147 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7 |
| Table of Contents |
|
SUMMARY OF RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. These risks are discussed more fully in the “Risk Factors” section immediately following this prospectus summary. These risks include, but are not limited to, the following:
Risks Related to Our Business and Industry |
||
|
|
|
|
|
| · | Our business could be materially adversely impacted by the COVID-19 pandemic. |
|
|
|
|
|
| · | Implementation of technology initiatives could disrupt our operations in the near term and fail to provide the anticipated benefits. |
|
|
|
|
|
| · | If our information technology systems suffer interruptions or failures, including as a result of cyber-attacks, our business operations could be disrupted and our reputation could suffer. |
|
|
|
|
|
| · | We rely on software and services from other parties. Defects in or the loss of access to software or services from third parties could increase our costs and adversely affect the quality of our products. |
|
|
|
|
|
| · | Failure to comply with data privacy and security laws and regulations could adversely affect our operating results and business. |
|
|
|
|
|
| · | Risks Related to This Offering and Ownership of Our Common Stock |
|
|
|
|
|
| · | The market price of our common stock may fluctuate, and you could lose all or part of your investment. |
|
|
|
|
|
| · | We may not be able to maintain a listing of our common stock on the NYSE American. |
|
|
|
|
|
| · | We have considerable discretion as to the use of the net proceeds from this offering and we may use these proceeds in ways with which you may not agree. |
|
|
|
|
|
| · | You will experience immediate and substantial dilution as a result of this offering. |
|
|
|
|
|
| · | We do not expect to declare or pay dividends in the foreseeable future. |
|
|
|
|
|
| · | Future issuances of our common stock or securities convertible into, or exercisable or exchangeable for, our common stock, or the expiration of lock-up agreements that restrict the issuance of new common stock or the trading of outstanding common stock, could cause the market price of our securities to decline and would result in the dilution of your holdings. |
|
|
|
|
|
| · | Future issuances of debt securities, which would rank senior to our common stock upon our bankruptcy or liquidation, and future issuances of preferred stock, which could rank senior to our common stock for the purposes of dividends and liquidating distributions, may adversely affect the level of return you may be able to achieve from an investment in our common stock. |
|
|
|
|
| 8 |
| Table of Contents |
An investment in our common stock involves a high degree of risk. You should carefully consider the following risk factors, together with the other information contained in this prospectus, before purchasing our common stock. We have listed below (not necessarily in order of importance or probability of occurrence) what we believe to be the most significant risk factors applicable to us, but they do not constitute all of the risks that may be applicable to us. Any of the following factors could harm our business, financial condition, results of operations or prospects, and could result in a partial or complete loss of your investment. Some statements in this prospectus, including statements in the following risk factors, constitute forward-looking statements. Please refer to the section titled “Cautionary Statement Regarding Forward-Looking Statements.”
Risks Related to Our Business and Industry
The near-term effects of the recent COVID-19 coronavirus pandemic are known, as they adversely affected our business. Some longer term effects, such as supply chain issues and inflation, are becoming known and may adversely affect our business, results of operations, financial condition, liquidity and cash flow.
Over the past two years the impact of COVID-19 has had adverse effects on our business by slowing down our ability to work with third parties outside of Seattle on testing and validation. We have witnessed supply chain related delays and increasing costs due to inflation. It is difficult to predict what other adverse effects, if any, COVID-19 and related matters can have on our business, or against the various aspects of same.
On January 30, 2020, the World Health Organization (“WHO”) announced a global health emergency caused by a new strain of the coronavirus (“COVID-19”) and advised of the risks to the international community as the virus spread globally. In March 2020, the WHO classified the COVID-19 outbreak as a pandemic based on the rapid increase in exposure globally. The spread of COVID-19 coronavirus caused public health officials to recommend precautions to mitigate the spread of the virus, especially as to travel and congregating in large numbers. Over time, the incidence of COVID-19 and its variants has diminished although periodic spikes in incidence occur. Consequently, restrictions imposed by various governmental health organizations may change over time. Several states have lifted restrictions only to reimpose such restrictions as the number of cases rise and new variants arise.
It is difficult to isolate the impact of the pandemic on our business, results of operations, financial condition and our future strategic plans.
The Company may experience long-term disruptions to its operations resulting from changes in government policy or guidance; quarantines of employees, customers and suppliers in areas affected by the pandemic and the presence of new variants of COVID-19; and closures of businesses or manufacturing facilities critical to its business or supply chains. The Company is actively monitoring, and will continue to actively monitor, the pandemic and the potential impact on its operations, financial condition, liquidity, suppliers, industry and workforce.
General securities market uncertainties resulting from the COVID-19 pandemic.
Since the outset of the pandemic the United States and worldwide national securities markets have undergone unprecedented stress due to the uncertainties of the pandemic and the resulting reactions and outcomes of government, business and the general population. These uncertainties have resulted in declines in all market sectors, increases in volumes due to flight to safety and governmental actions to support the markets. As a result, until the pandemic has stabilized, the markets may not be available to the Company for purposes of raising required capital. Should we not be able to obtain financing when required, in the amounts necessary to execute on our plans in full, or on terms which are economically feasible we may be unable to sustain the necessary capital to pursue our strategic plan and may have to reduce the planned future growth and/or scope of our operations.
General securities market uncertainties resulting in geo-political considerations.
Since the outset of the military conflict in Ukraine, the United States and worldwide national securities markets have undergone unprecedented stress due to the uncertainties of that conflict and the resulting reactions and outcomes of governments, businesses, and the general population. These uncertainties have resulted in declines in all market sectors, increases in volumes due to flight to safety and governmental actions to support the markets. As a result, until the military conflict has stabilized, the markets may not be available to the Company for purposes of raising required capital. Should we not be able to obtain financing when required, in the amounts necessary to execute on our plans in full, or on terms which are economically feasible, we may be unable to sustain the necessary capital to pursue our strategic plan and may have to reduce the planned future growth and/or scope of our operations.
| 9 |
| Table of Contents |
General securities market uncertainties resulting in economic considerations.
Recent unease regarding the aforementioned geo-political considerations and increasing inflation has caused the United States and worldwide national securities markets to have undergone unprecedented stress due to the uncertainties of regarding the economy and the resulting reactions and outcomes of governments, businesses, and the general population. These uncertainties have resulted in declines in all market sectors, increases in volumes due to flight to safety and governmental actions to support the markets. As a result, until economic outlook has stabilized, the markets may not be available to the Company for purposes of raising required capital. Should we not be able to obtain financing when required, in the amounts necessary to execute on our plans in full, or on terms which are economically feasible, we may be unable to sustain the necessary capital to pursue our strategic plan and may have to reduce the planned future growth and/or scope of our operations.
We need additional financing to support our technology development and ongoing operations, pay our debts and maintain ownership of our intellectual properties.
We are currently operating at a loss and using substantial cash to fund our operation. We believe that our cash on hand will be sufficient to fund our operations through June 30, 2023. We may need additional financing to implement our business plan and to service our ongoing operations, pay our current debts (described below) and maintain ownership of our intellectual property. There can be no assurance that we will be able to secure any needed funding, or that if such funding is available, the terms or conditions would be acceptable to us. If we are unable to obtain additional financing when it is needed, we will need to restructure our operations and/or divest all or a portion of our business. We are each seeking additional capital through a combination of private and public equity offerings, debt financings and strategic collaborations. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, and could increase our expenses and require that our assets secure such debt. Equity financing, if obtained, could result in dilution to our then-existing stockholders and/or require such stockholders to waive certain rights and preferences. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back, eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected. There can be no assurance that we will be able to sell that number of shares, if any.
We need to continue as a going concern if our business is to succeed.
We have cash and cash equivalents of $8,351,945 and net working capital of approximately $6,042,000 (exclusive of convertible notes payable) as of June 30, 2022. We anticipate that it will record losses from operations for the foreseeable future. We believe that we have enough available cash to operate until June 30, 2023. As of June 30, 2022, our accumulated deficit was $95,827,137. We have limited capital resources and intends to seek additional cash via equity and debt offerings.
On July 29, 2022, we filed a Form S-1 registration statement, of which this prospectus forms a part, for this new offering of 3 million shares of our common stock with a public offering price of $2.00 per share.
The proceeds of warrants currently outstanding, which could be exercised on a cash basis, may generate potential proceeds of up to $16,383,908. We cannot provide assurance that any of these warrants will be exercised.
If this offering is unsuccessful or the warrants are not exercised for cash, the cash conditions may raise substantial doubt about our ability to continue as a going concern. The audit report prepared by our independent registered public accounting firm relating to our consolidated financial statements for the year ended September 30, 2021 did not include an explanatory paragraph expressing the substantial doubt about the our ability to continue as a going concern.
As of June 30, 2022, we owed approximately $2,529,706 and if we do not satisfy these obligations, the lenders may have the right to demand payment in full or exercise other remedies.
Mr. Erickson, our Chairman, and/or entities with which he is affiliated also have accounts payable and accrued liabilities $274,640 of as of June 30, 2022 related to accrued compensation, accrued interest and expenses.
We owe $2,255,066 under various convertible promissory notes as of June 30, 2022 including $1,184,066 owed to entities controlled by our Chairman.
We may need additional financing, to service and/or repay these debt obligations. If we raise additional capital through borrowing or other debt financing, we may incur substantial interest expense. If and when we raise more equity capital in the future, it will result in substantial dilution to our current stockholders.
| 10 |
| Table of Contents |
We have a history of operating losses and there can be no assurance that we can achieve or maintain profitability.
We have experienced net losses since inception. As of June 30, 2022, we had an accumulated deficit of $95,827,137 and net losses in the amount of $25,360,213, and $13,562,641 for the years ended September 30, 2021 and 2020, respectively, and $14,500,643 and $17,763,812 for the nine months ended June 30, 2022 and 2021, respectively.
There can be no assurance that we will achieve or maintain profitability. If we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Failure to become and remain profitable would impair our ability to sustain operations and adversely affect the price of our common stock and our ability to raise capital. Our operating expenses may increase as we spend resources on growing our business, and if our revenue does not correspondingly increase, our operating results and financial condition will suffer. Our Know Labs, Particle, and AI Mind businesses have produced minimal revenues and may not produce significant revenues in the near term, or at all, which would harm our ability to continue our operations or obtain additional financing and require us to reduce or discontinue our operations. You must consider our business and prospects in light of the risks and difficulties we will encounter as business with an early-stage technology in a new and rapidly evolving industry. We may not be able to successfully address these risks and difficulties, which could significantly harm our business, operating results and financial condition.
If our company were to dissolve or wind-up operations, holders of our common stock would not receive a liquidation preference.
If we were to wind-up or dissolve our Company and liquidate and distribute our assets, our common stockholders would share in our assets only after we satisfy any amounts we owe to our creditors and preferred equity holders. If our liquidation or dissolution were attributable to our inability to profitably operate our business, then it is likely that we would have material liabilities at the time of liquidation or dissolution. Accordingly, it is very unlikely that sufficient assets will remain available after the payment of our creditors and preferred equity holders to enable common stockholders to receive any liquidation distribution with respect to any common stock.
We may not be able to generate sufficient revenue from the commercialization of our technology and related products to achieve or sustain profitability.
We are in the early stages of commercializing our technology. Failure to develop and sell products based upon our technology, grant additional licenses and obtain royalties or develop other revenue streams will have a material adverse effect on our business, financial condition and results of operations.
To date, we have generated minimal revenue from sales of our products. We believe that our commercialization success is dependent upon our ability to significantly increase the number of customers that are using our products. In addition, demand for our products may not materialize, or increase as quickly as planned, and we may therefore be unable to increase our revenue levels as expected. We are currently not profitable. Even if we succeed in introducing our technology and related products to our target markets, we may not be able to generate sufficient revenue to achieve or sustain profitability.
We currently rely in part upon external resources for engineering and product development services. If we are unable to secure an engineering or product development partner or establish satisfactory engineering and product development capabilities, we may not be able to successfully commercialize our technology.
Our success depends upon our ability to develop products that are accurate and provide solutions for our customers. Achieving the desired results for our customers requires solving engineering issues in concert with them. Any failure of our technology or related products to meet customer expectations could result in customers choosing to retain their existing methods or to adopt systems other than ours.
We have not historically had sufficient internal resources which can work on engineering and product development matters. We have used third parties in the past and will continue to do so. These resources are not always readily available, and the absence of their availability could inhibit our research and development efforts and our responsiveness to our customers. Our inability to secure those resources could impact our ability to provide engineering and product development services and could have an impact on our customers’ willingness to use our technology.
| 11 |
| Table of Contents |
We are in the early stages of commercialization and our technology and related products may never achieve significant commercial market acceptance.
Our success depends on our ability to develop and market products that are recognized as accurate and cost-effective. Many of our potential customers may be reluctant to use our new technology. Market acceptance will depend on many factors, including our ability to convince potential customers that our technology and related products are an attractive alternative to existing technologies. We will need to demonstrate that our products provide accurate and cost-effective alternatives to existing technologies. Compared to most competing technologies, our technology is relatively new, and most potential customers have limited knowledge of, or experience with, our products. Prior to implementing our technology and related products, some potential customers may be required to devote significant time and effort to testing and validating our products. In addition, during the implementation phase, some customers may be required to devote significant time and effort to training their personnel on appropriate practices to ensure accurate results from our technology and products. Any failure of our technology or related products to meet customer expectations could result in customers choosing to retain their existing testing methods or to adopt systems other than ours.
Many factors influence the perception of a system including its use by leaders in the industry. If we are unable to induce industry leaders in our target markets to implement and use our technology and related products, acceptance and adoption of our products could be slowed. In addition, if our products fail to gain significant acceptance in the marketplace and we are unable to expand our customer base, we may never generate sufficient revenue to achieve or sustain profitability.
Our management has had a material weakness in our internal controls over financial reporting and that our disclosure controls and procedures are not effective.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected on a timely basis. During the audit of our financial statements for the year ended September 30, 2021, management identified a material weakness during its assessment of internal controls over financial reporting. Specifically, we did not employ a full time Chief Financial Officer. Peter Conley was appointed as Chief Financial Officer on May 24, 2022.
Our Particle, Inc. subsidiary was incorporated April 30, 2020, and has limited operating history.
Particle, Inc., or Particle, was incorporated April 30, 2020, and to date has engaged in activities consisting primarily of research and development on threaded light bulbs that have a warm white light that can inactivate germs, including bacteria and viruses. On June 1, 2020, we approved and ratified entry into an intercompany Patent License Agreement dated May 21, 2020, with Particle. Pursuant to the Agreement, Particle received an exclusive non-transferrable license to use certain patents and trademarks of our company, in exchange our company shall receive: (i) a one-time fee of $250,000 upon a successful financing of Particle, and (ii) a quarterly royalty payment equal to the greater of 5% of the Gross Sales, net of returns, from Particle or $5,000. As of June 30, 2022, the operations of Particle have generated no sales and operations are just commencing. The first product, the Particle bulb can be used in households, businesses and other facilities to inactivate bacteria and viruses. Through internal preliminary testing, Particle personnel has confirmed the bulb’s efficacy in inactivating common germs such as E. coli and Staphylococcus. A world renowned, CDC-regulated biosafety level-4 laboratory has tested the Particle bulb’s ability to inactivate SARS-CoV-2, the virus that causes COVID-19. The results of these tests were successful, confirming the bulb’s ability to deactivate Alpha and Delta variants of the virus.
To date, we have generated no revenue from Particle. We may not generate revenues in the near future while products are being developed. We believe that Particle’s commercialization success is dependent upon its ability to develop successful products to take to market. In addition, once developed, demand for its products may not materialize, or increase as quickly as planned, and we may therefore be unable to increase our revenue levels as expected. Even if we succeed in introducing our technology and related products to our target markets, we may not be able to generate sufficient revenue to achieve or sustain profitability of the Particle subsidiary. Our company is also exploring strategic partnerships and distribution agreements for Particle. These efforts may not be successful which would adversely impact the sustainability of Particle.
Our AI Mind, Inc. subsidiary was incorporated on September 17, 2021, and has limited operating history.
The subsidiary, which is wholly owned by Know Labs, Inc. commenced activity in the last calendar quarter of 2021 and the first quarter of our 2022 fiscal year. It has generated its first revenues during the first quarter of fiscal 2022 time from its initial commercialization efforts related to the generation of NFT’s. There can be no assurance that it will continue to generate revenues nor be successful in continuing its marketing of parent company assets. These assets rely on fundamental trade secrets which at this time are proprietary yet not protected by any pending patents. It may not be possible to protect these trade secrets which would impact the ability of AI Mind to continue to generate revenues.
| 12 |
| Table of Contents |
We are dependent on key personnel.
Our success depends to a significant degree upon the continued contributions of key management and other personnel, some of whom could be difficult to replace, including Ronald P. Erickson, our Chairman and Phil Bosua, our Chief Executive Officer. We maintain key person life insurance on our Chief Executive Officer, Phil Bosua. Our success will depend on the performance of our officers, our ability to retain and motivate our officers, our ability to integrate new officers into our operations, and the ability of all personnel to work together effectively as a team. Our failure to retain and recruit officers and other key personnel could have a material adverse effect on our business, financial condition and results of operations. Our success also depends on our continued ability to identify, attract, hire, train, retain and motivate highly skilled technical, managerial, manufacturing, administrative and sales and marketing personnel. Competition for these individuals is intense, and we may not be able to successfully recruit, assimilate or retain sufficiently qualified personnel. In particular, we may encounter difficulties in recruiting and retaining a sufficient number of qualified technical personnel, which could harm our ability to develop new products and adversely impact our relationships with existing and future customers. The inability to attract and retain necessary technical, managerial, manufacturing, administrative and sales and marketing personnel could harm our ability to obtain new customers and develop new products and could adversely affect our business and operating results.
We have limited insurance which may not cover claims by third parties against us or our officers and directors.
We have limited directors’ and officers’ liability insurance and commercial liability insurance policies. Claims by third parties against us may exceed policy amounts and we may not have amounts to cover these claims. Any significant claims would have a material adverse effect on our business, financial condition and results of operations. In addition, our limited directors’ and officers’ liability insurance may affect our ability to attract and retain directors and officers.
Our inability to effectively protect our intellectual property would adversely affect our ability to compete effectively, our revenue, our financial condition and our results of operations.
We rely on a combination of patent, trademark, and trade secret laws, confidentiality procedures and licensing arrangements to protect our intellectual property rights. Obtaining and maintaining a strong patent position is important to our business. Patent law relating to the scope of claims in the technology fields in which we operate is complex and uncertain, so we cannot be assured that we will be able to obtain or maintain patent rights, or that the patent rights we may obtain will be valuable, provide an effective barrier to competitors or otherwise provide competitive advantages. Others have filed, and in the future are likely to file, patent applications that are similar or identical to ours or those of our licensors. To determine the priority of inventions or demonstrate that we did not derive our invention from another, we may have to participate in interference or derivation proceedings in the USPTO or in court that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. We cannot be assured our patent applications will prevail over those filed by others. Also, our intellectual property rights may be subject to other challenges by third parties. Patents we obtain could be challenged in litigation or in administrative proceedings such as ex parte reexam, inter parties review, or post grant review in the United States or opposition proceedings in Europe or other jurisdictions.
There can be no assurance that:
|
| · | any of our existing patents will continue to be held valid, if challenged; |
|
|
|
|
|
| · | patents will be issued for any of our pending applications; |
|
|
|
|
|
| · | any claims allowed from existing or pending patents will have sufficient scope or strength to protect us; |
|
|
|
|
|
| · | our patents will be issued in the primary countries where our products are sold in order to protect our rights and potential commercial advantage; or |
|
|
|
|
|
| · | any of our products or technologies will not infringe on the patents of other companies. |
If we are enjoined from selling our products, or if we are required to develop new technologies or pay significant monetary damages or are required to make substantial royalty payments, our business and results of operations would be harmed.
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
| 13 |
| Table of Contents |
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or interferences against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could have a material adverse effect on our results of operations and business.
Claims by others that our products infringe their patents or other intellectual property rights could prevent us from manufacturing and selling some of our products or require us to pay royalties or incur substantial costs from litigation or development of non-infringing technology.
In recent years, there has been significant litigation in the United States involving patents and other intellectual property rights. We may receive notices that claim we have infringed upon the intellectual property of others. Even if these claims are not valid, they could subject us to significant costs. Any such claims, with or without merit, could be time-consuming to defend, result in costly litigation, divert our attention and resources, cause product shipment delays or require us to enter into royalty or licensing agreements. Such royalty or licensing agreements, if required, may not be available on terms acceptable to us or at all. We have not been engaged in litigation but litigation may be necessary in the future to enforce our intellectual property rights or to determine the validity and scope of the proprietary rights of others. Litigation may also be necessary to defend against claims of infringement or invalidity by others. A successful claim of intellectual property infringement against us and our failure or inability to license the infringed technology or develop or license technology with comparable functionality could have a material adverse effect on our business, financial condition and operating results.
If we are unable to secure a sales and marketing partner or establish satisfactory sales and marketing capabilities at our company, we may not be able to successfully commercialize our technology.
If we are not successful entering into appropriate collaboration arrangements or recruiting sales and marketing personnel or in building a sales and marketing infrastructure, we will have difficulty successfully commercializing our technology, which would adversely affect our business, operating results and financial condition.
We may not be able to enter into collaboration agreements on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. If we elect to establish a sales and marketing infrastructure, we may not realize a positive return on this investment. In addition, we must compete with established and well-funded pharmaceutical and biotechnology companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize technology without strategic partners or licensees include:
|
| · | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
|
|
|
|
|
| · | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
|
|
|
|
|
| · | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
Government regulatory approval may be necessary before some of our products can be sold and there is no assurance such approval will be granted.
Our technology may have a number of potential applications in fields of use which will require prior governmental regulatory approval before the technology can be introduced to the marketplace. For example, we are exploring the use of our technology for certain medical diagnostic applications, with an initial focus on the monitoring of blood glucose. There is no assurance that we will be successful in developing glucose monitoring medical applications for our technology. If we were to be successful in developing glucose monitoring medical applications of our technology, prior clearance by the FDA and other governmental regulatory bodies will be required before the technology could be introduced into the marketplace. There is no assurance that such regulatory approval would be obtained for a glucose monitoring medical diagnostic device or other applications requiring such approval. The FDA can refuse to grant, delay, and limit or deny approval of an application for clearance of marketing a glucose monitoring device for many reasons. We may not obtain the necessary regulatory approvals or clearances to market these glucose monitoring systems in the United States or outside of the United States. Any delay in, or failure to receive or maintain, approval or clearance for our products could prevent us from generating revenue from these products or achieving profitability.
| 14 |
| Table of Contents |
Cybersecurity risks and cyber incidents could result in the compromise of confidential data or critical data systems and give rise to potential harm to customers, remediation and other expenses, expose us to liability under HIPAA, consumer protection laws, or other common law theories, subject us to litigation and federal and state governmental inquiries, damage our reputation, and otherwise be disruptive to our business and operations.
Cyber incidents can result from deliberate attacks or unintentional events. We collect and store on our networks sensitive information, including intellectual property, proprietary business information and personally identifiable information of our customers. The secure maintenance of this information and technology is critical to our business operations. We have implemented multiple layers of security measures to protect the confidentiality, integrity and availability of this data and the systems and devices that store and transmit such data. We utilize current security technologies, and our defenses are monitored and routinely tested internally and by external parties. Despite these efforts, threats from malicious persons and groups, new vulnerabilities and advanced new attacks against information systems create risk of cybersecurity incidents. These incidents can include, but are not limited to, gaining unauthorized access to digital systems for purposes of misappropriating assets or sensitive information, corrupting data, or causing operational disruption. Because the techniques used to obtain unauthorized access, disable or degrade service, or sabotage systems change frequently and may not immediately produce signs of intrusion, we may be unable to anticipate these incidents or techniques, timely discover them, or implement adequate preventative measures.
These threats can come from a variety of sources, ranging in sophistication from an individual hacker to malfeasance by employees, consultants or other service providers to state-sponsored attacks. Cyber threats may be generic, or they may be custom crafted against our information systems. Over the past several years, cyber-attacks have become more prevalent and much harder to detect and defend against. Our network and storage applications may be vulnerable to cyber-attack, malicious intrusion, malfeasance, loss of data privacy or other significant disruption and may be subject to unauthorized access by hackers, employees, consultants or other service providers. In addition, hardware, software or applications we develop or procure from third parties may contain defects in design or manufacture or other problems that could unexpectedly compromise information security. Unauthorized parties may also attempt to gain access to our systems or facilities through fraud, trickery or other forms of deceiving our employees, contractors and temporary staff.
There can be no assurance that we will not be subject to cybersecurity incidents that bypass our security measures, impact the integrity, availability or privacy of personal health information or other data subject to privacy laws or disrupt our information systems, devices or business, including our ability to deliver services to our customers. As a result, cybersecurity, physical security and the continued development and enhancement of our controls, processes and practices designed to protect our enterprise, information systems and data from attack, damage or unauthorized access remain a priority for us. As cyber threats continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any cybersecurity vulnerabilities.
We may engage in acquisitions, mergers, strategic alliances, joint ventures and divestures that could result in final results that are different than expected.
In the normal course of business, we engage in discussions relating to possible acquisitions, equity investments, mergers, strategic alliances, joint ventures and divestitures. Such transactions are accompanied by a number of risks, including the use of significant amounts of cash, potentially dilutive issuances of equity securities, incurrence of debt on potentially unfavorable terms as well as impairment expenses related to goodwill and amortization expenses related to other intangible assets, the possibility that we may pay too much cash or issue too many of our shares as the purchase price for an acquisition relative to the economic benefits that we ultimately derive from such acquisition, and various potential difficulties involved in integrating acquired businesses into our operations.
From time to time, we have also engaged in discussions with candidates regarding the potential acquisitions of our product lines, technologies and businesses. If a divestiture such as this does occur, we cannot be certain that our business, operating results and financial condition will not be materially and adversely affected. A successful divestiture depends on various factors, including our ability to effectively transfer liabilities, contracts, facilities and employees to any purchaser; identify and separate the intellectual property to be divested from the intellectual property that we wish to retain; reduce fixed costs previously associated with the divested assets or business; and collect the proceeds from any divestitures.
If we do not realize the expected benefits of any acquisition or divestiture transaction, our financial position, results of operations, cash flows and stock price could be negatively impacted.
| 15 |
| Table of Contents |
We have made strategic acquisitions in the past and may do so in the future, and if the acquired companies do not perform as expected, this could adversely affect our operating results, financial condition and existing business.
We may continue to expand our business through strategic acquisitions. The success of any acquisition will depend on, among other things:
|
| · | the availability of suitable candidates; |
|
|
|
|
|
| · | higher than anticipated acquisition costs and expenses; |
|
|
|
|
|
| · | competition from other companies for the purchase of available candidates; |
|
|
|
|
|
| · | our ability to value those candidates accurately and negotiate favorable terms for those acquisitions; |
|
|
|
|
|
| · | the availability of funds to finance acquisitions and obtaining any consents necessary under our credit facility; |
|
|
|
|
|
| · | the ability to establish new informational, operational and financial systems to meet the needs of our business; |
|
|
|
|
|
| · | the ability to achieve anticipated synergies, including with respect to complementary products or services; and |
|
|
|
|
|
| · | the availability of management resources to oversee the integration and operation of the acquired businesses. |
We may not be successful in effectively integrating acquired businesses and completing acquisitions in the future. We also may incur substantial expenses and devote significant management time and resources in seeking to complete acquisitions. Acquired businesses may fail to meet our performance expectations. If we do not achieve the anticipated benefits of an acquisition as rapidly as expected, or at all, investors or analysts may not perceive the same benefits of the acquisition as we do. If these risks materialize, our stock price could be materially adversely affected.
We are subject to corporate governance and internal control requirements, and our costs related to compliance with, or our failure to comply with existing and future requirements could adversely affect our business.
We must comply with corporate governance requirements under the Sarbanes-Oxley Act of 2002 and the Dodd–Frank Wall Street Reform and Consumer Protection Act of 2010, as well as additional rules and regulations currently in place and that may be subsequently adopted by the SEC and the Public Company Accounting Oversight Board. These laws, rules, and regulations continue to evolve and may become increasingly stringent in the future. The financial cost of compliance with these laws, rules, and regulations is expected to remain substantial.
We cannot assure you that we will be able to fully comply with these laws, rules, and regulations that address corporate governance, internal control reporting, and similar matters in the future. Failure to comply with these laws, rules and regulations could materially adversely affect our reputation, financial condition, and the value of our securities.
The exercise prices of certain warrants, and the conversion prices of our outstanding convertible notes payable and our Series C and D Preferred Shares may require further adjustment.
If in the future, if we sell our common stock at a price below $0.25 per share, the conversion price of 8,108,356 outstanding shares of Series C and D Preferred Stock would adjust below $0.25 per share pursuant to the documents governing such instruments. In addition, the conversion price of Convertible Notes Payable in the principal amount of $2,255,066, that convert into 9,020,264 shares of our common stock at $0.25 per share and the exercise price of certain outstanding warrants to purchase 10,284,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments. Warrants totaling 4,487,207 would adjust below $1.20 per share and warrants totaling 3,954,625 would adjust below $2.40 per share, in each case pursuant to the documents governing such instruments.
| 16 |
| Table of Contents |
We or our manufacturers may be unable to obtain or maintain international regulatory clearances or approvals for our current or future products, or our distributors may be unable to obtain necessary qualifications, which could harm our business.
Sales of the Know Labs products internationally are subject to foreign regulatory requirements that vary widely from country to country. In addition, the FDA regulates exports of medical devices from the U.S. Complying with international regulatory requirements can be an expensive and time-consuming process, and marketing approval or clearance is not certain. The time required to obtain clearances or approvals, if required by other countries, may be longer than that required for FDA clearance or approvals, and requirements for such clearances or approvals may significantly differ from FDA requirements. We may rely on third-party distributors to obtain regulatory clearances and approvals required in other countries, and these distributors may be unable to obtain or maintain such clearances or approvals. Our distributors may also incur significant costs in attempting to obtain and in maintaining foreign regulatory approvals or clearances, which could increase the difficulty of attracting and retaining qualified distributors. If our distributors experience delays in receiving necessary qualifications, clearances or approvals to market our products outside the U.S., or if they fail to receive those qualifications, clearances or approvals, we may be unable to market our products or enhancements in international markets effectively, or at all.
Foreign governmental authorities that regulate the manufacture and sale of medical devices have become increasingly stringent and, to the extent we market and sell our products outside of the U.S., we may be subject to rigorous international regulation in the future. In these circumstances, we would be required to rely on our foreign independent distributors to comply with the varying regulations, and any failures on their part could result in restrictions on the sale of our product in foreign countries.
Risks Relating to this Offering and Ownership of Our Common Stock
We may not be able to maintain a listing of our common stock on NYSE American.
Prior to this offering, our common stock was traded on the OTCQB under the symbol “KNWN.” In connection with this offering, we received approval from NYSE American for the listing of our common stock under the symbol “KNW.” Our common stock will commence trading on September 16, 2022. We must meet certain financial and liquidity criteria to maintain the listing of our common stock on NYSE American. If we fail to meet any listing standards or if we violate any listing requirements, our common stock may be delisted. In addition, our board of directors may determine that the cost of maintaining our listing on a national securities exchange outweighs the benefits of such listing. A delisting of our common stock from NYSE American may materially impair our stockholders’ ability to buy and sell our common stock and could have an adverse effect on the market price of, and the efficiency of the trading market for, our common stock. The delisting of our common stock could significantly impair our ability to raise capital and the value of your investment.
The price of our common stock is volatile, which may cause investment losses for our stockholders.
The market price of our common stock has been and is likely in the future to be volatile. Our common stock price may fluctuate in response to factors such as:
|
| · | Announcements by us regarding liquidity, significant acquisitions, equity investments and divestitures, strategic relationships, addition or loss of significant customers and contracts, capital expenditure commitments and litigation; |
|
|
|
|
|
| · | Issuance of convertible or equity securities and related warrants for general or merger and acquisition purposes; |
|
|
|
|
|
| · | Issuance or repayment of debt, accounts payable or convertible debt for general or merger and acquisition purposes; |
|
|
|
|
|
| · | Sale of a significant number of shares of our common stock by stockholders; |
|
|
|
|
|
| · | General market and economic conditions; |
|
|
|
|
|
| · | Quarterly variations in our operating results; |
|
|
|
|
|
| · | Investor and public relation activities; |
|
|
|
|
|
| · | Announcements of technological innovations; |
|
|
|
|
|
| · | New product introductions by us or our competitors; |
|
|
|
|
|
| · | Competitive activities; |
|
|
|
|
|
| · | Low liquidity; and |
|
|
|
|
|
| · | Additions or departures of key personnel. |
| 17 |
| Table of Contents |
These broad market and industry factors may have a material adverse effect on the market price of our common stock, regardless of our actual operating performance. These factors could have a material adverse effect on our business, financial condition, and results of operations.
We have considerable discretion as to the use of the net proceeds from this offering and we may use these proceeds in ways with which you may not agree.
We intend to use the proceeds from this offering to make acquisitions and obtain partnerships, invest in technology, expand our sales team and marketing efforts, and general working capital and other corporate purposes. However, we have considerable discretion in the application of the proceeds. Because of the number and variability of factors that will determine our use of our net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. You will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the net proceeds of this offering. The net proceeds may be used for corporate or other purposes with which you do not agree or that do not improve our profitability or increase our share price. The net proceeds from this offering may also be placed in investments that do not produce income or that lose value. Please see “Use of Proceeds” below for more information.
You will experience immediate and substantial dilution as a result of this offering.
As of June 30, 2022, our net tangible book value was approximately $4,919,268 or approximately $0.11 per share. Since the effective price per share of our common stock being offered in this offering is substantially higher than the net tangible book value per share of our common stock, you will suffer substantial dilution with respect to the net tangible book value of the common stock that you purchase in this offering. Based on the public offering price of $2.00 per share of common stock being sold in this offering and our net tangible book value per share as of June 30, 2022, if you purchase shares in this offering, you will suffer immediate and substantial dilution of $1.76 per share (or $1.74 per share if the underwriters exercise the over-allotment option to purchase additional shares of common stock in full). See the section titled “Dilution” for a more detailed discussion of the dilution you will incur if you purchase shares in this offering.
The sale of a significant number of our shares of common stock could depress the price of our common stock.
As of June 30, 2022, we had 43,802,147 shares of common stock issued and outstanding. As of June 30, 2022, there were options outstanding for the purchase of 20,927,370 common shares (including unearned stock option grants totaling 11,550,745 shares related to performance targets), warrants for the purchase of 21,651,513 common shares, and 8,108,356 shares of our common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock. In addition, we currently have 9,020,264 common shares at the current price of $0.25 per share reserved and are issuable upon conversion of convertible debentures of $2,255,066. All of which could potentially dilute future earnings per share but are excluded from the June 30, 2022, calculation of net loss per share because their impact is antidilutive.
Significant shares of common stock are held by our principal stockholders, other company insiders and other large stockholders. As “affiliates,” as defined under Rule 144 under the Securities Act, our principal stockholders, other of our insiders and other large stockholders may only sell their shares of common stock in the public market pursuant to an effective registration statement or in compliance with Rule 144.
These options, warrants, convertible notes payable and convertible preferred stock could result in further dilution to common stockholders and may affect the market price of the common stock.
Future issuance of additional shares of common stock and/or preferred stock could dilute existing stockholders. We have and may issue preferred stock that could have rights that are preferential to the rights of common stock that could discourage potentially beneficial transactions to our common stockholders.
| 18 |
| Table of Contents |
Pursuant to our articles of incorporation, we currently have authorized 200,000,000 shares of common stock and 5,000,000 shares of preferred stock. To the extent that common shares are available for issuance, subject to compliance with applicable stock exchange listing rules, our board of directors has the ability to issue additional shares of common stock in the future for such consideration as the board of directors may consider sufficient. The issuance of any additional securities could, among other things, result in substantial dilution of the percentage ownership of our stockholders at the time of issuance, result in substantial dilution of our earnings per share and adversely affect the prevailing market price for our common stock.
An issuance of additional shares of preferred stock could result in a class of outstanding securities that would have preferences with respect to voting rights and dividends and in liquidation over our common stock and could, upon conversion or otherwise, have all of the rights of our common stock. Our board of directors’ authority to issue preferred stock could discourage potential takeover attempts or could delay or prevent a change in control through merger, tender offer, proxy contest or otherwise by making these attempts more difficult or costly to achieve. The issuance of preferred stock could impair the voting, dividend and liquidation rights of common stockholders without their approval.
Our articles of incorporation allow for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock.
Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our board of directors also has the authority to issue preferred stock without further stockholder approval. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders.
Future issuances of debt securities, which would rank senior to our common stock upon our bankruptcy or liquidation, and future issuances of preferred stock, which could rank senior to our common stock for the purposes of dividends and liquidating distributions, may adversely affect the level of return you may be able to achieve from an investment in our securities.
In the future, we may attempt to increase our capital resources by offering debt securities. Upon bankruptcy or liquidation, holders of our debt securities, and lenders with respect to other borrowings we may make, would receive distributions of our available assets prior to any distributions being made to holders of our common stock. Moreover, if we issue preferred stock, the holders of such preferred stock could be entitled to preferences over holders of common stock in respect of the payment of dividends and the payment of liquidating distributions. Because our decision to issue debt or preferred stock in any future offering, or borrow money from lenders, will depend in part on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of any such future offerings or borrowings. Holders of our securities must bear the risk that any future offerings we conduct or borrowings we make may adversely affect the level of return, if any, they may be able to achieve from an investment in our securities.
Future capital raises may dilute our existing stockholders’ ownership and/or have other adverse effects on our operations.
If we raise additional capital by issuing equity securities, our existing stockholders’ percentage ownership will be reduced, and these stockholders may experience substantial dilution. We may also issue equity securities that provide for rights, preferences and privileges senior to those of our common stock. If we raise additional funds by issuing debt securities, these debt securities would have rights senior to those of our common stock and the terms of the debt securities issued could impose significant restrictions on our operations, including liens on our assets. If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our technologies or candidate products, or to grant licenses on terms that are not favorable to us.
We do not anticipate paying any cash dividends on our capital stock in the foreseeable future.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business, and we do not anticipate paying any cash dividends on our capital stock in the foreseeable future. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
If securities industry analysts do not publish research reports on us, or publish unfavorable reports on us, then the market price and market trading volume of our common stock could be negatively affected.
Any trading market for our common stock may be influenced in part by any research reports that securities industry analysts publish about us. We do not currently have and may never obtain research coverage by securities industry analysts. If no securities industry analysts commence coverage of us, the market price and market trading volume of our securities could be negatively affected. In the event we are covered by analysts, and one or more of such analysts downgrade our securities, or otherwise reports on us unfavorably, or discontinues coverage of us, the market price and market trading volume of our securities could be negatively affected.
| 19 |
| Table of Contents |
If our securities become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not retain a listing on NYSE American or another national securities exchange and if the price of our securities is less than $5.00, our securities could be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our securities, and therefore stockholders may have difficulty selling their securities.
Anti-takeover provisions may limit the ability of another party to acquire our company, which could cause our stock price to decline.
Our articles of incorporation, our bylaws and Nevada law contain provisions that could discourage, delay or prevent a third party from acquiring our company, even if doing so may be beneficial to our stockholders. In addition, these provisions could limit the price investors would be willing to pay in the future for shares of our common stock.
We are authorized to issue “blank check” preferred stock without stockholder approval, which could adversely impact the rights of holders of our securities.
Our articles of incorporation authorize us to issue up to 5,000,000 shares of blank check preferred stock. Any preferred stock that we issue in the future may rank ahead of our securities in terms of dividend priority or liquidation premiums and may have greater voting rights than our securities. In addition, such preferred stock may contain provisions allowing those shares to be converted into shares of common stock, which could dilute the value of our securities to current stockholders and could adversely affect the market price, if any, of our securities. In addition, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of our company. Although we have no present intention to issue any shares of authorized preferred stock, there can be no assurance that we will not do so in the future.
| 20 |
| Table of Contents |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to us. All statements other than statements of historical facts are forward-looking statements. The forward-looking statements are contained principally in, but not limited to, the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
|
| · | our goals and strategies; |
|
|
|
|
|
| · | our future business development, financial condition and results of operations; |
|
|
|
|
|
| · | expected changes in our revenue, costs or expenditures; |
|
|
|
|
|
| · | growth of and competition trends in our industry; |
|
|
|
|
|
| · | our expectations regarding demand for, and market acceptance of, our products; |
|
|
|
|
|
| · | our expectations regarding our relationships with investors, institutional funding partners and other parties with whom we collaborate; |
|
|
|
|
|
| · | our expectation regarding the use of proceeds from this offering; |
|
|
|
|
|
| · | fluctuations in general economic and business conditions in the markets in which we operate; and |
|
|
|
|
|
| · | relevant government policies and regulations relating to our industry. |
In some cases, you can identify forward-looking statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the heading “Risk Factors” and elsewhere in this prospectus. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance.
The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Although we will become a public company after this offering and have ongoing disclosure obligations under United States federal securities laws, we do not intend to update or otherwise revise the forward-looking statements in this prospectus, whether as a result of new information, future events or otherwise.
| 21 |
| Table of Contents |
After deducting the estimated underwriters’ discounts and commissions and offering expenses payable by us, we expect to receive net proceeds of approximately $6.35 million from this offering (or approximately $7.37 million if the underwriters exercise the over-allotment option in full).
We plan to use the net proceeds of this offering as follows:
(dollars in thousands)
| Research and development expenses |
| $ | 1,767 |
|
| Sales and marketing |
|
| 1,325 |
|
| General and administrative |
|
| 883 |
|
| Capital investments |
|
| 442 |
|
| Working Capital |
|
| 1,930 |
|
|
|
| $ | 6,347 |
|
The foregoing represents our current intentions to use and allocate the net proceeds of this offering based upon our present plans and business conditions. Our management, however, will have broad discretion in the way that we use the net proceeds of this offering. Pending the final application of the net proceeds of this offering, we intend to invest the net proceeds of this offering in short-term, interest-bearing, investment-grade securities. See “Risk Factors—Risks Related to This Offering and Ownership of Our Common Stock—We have considerable discretion as to the use of the net proceeds from this offering and we may use these proceeds in ways with which you may not agree.”
| 22 |
| Table of Contents |
We have never declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any cash dividends on our common stock in the near future. We may also enter into credit agreements or other borrowing arrangements in the future that will restrict our ability to declare or pay cash dividends on our common stock. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, contractual restrictions, general business conditions and other factors that our board of directors may deem relevant. See also “Risk Factors—Risks Related to This Offering and Ownership of Our Common Stock—We do not expect to declare or pay dividends in the foreseeable future.”
| 23 |
| Table of Contents |
The following table sets forth our capitalization as of June 30, 2022:
|
| · | on an actual basis; and |
|
|
|
|
|
| · | on an as adjusted basis to reflect the issuance of 47,134 shares of common stock subsequent to June 30, 2022 and the sale of 3,600,000 shares by us in this offering at a public offering price of $2.00 per share, resulting in net proceeds to us of $6,347,000 after deducting underwriter commissions of $504,000, the non-accountable expense allowance of $72,000 and our estimated other offering expenses of $277,000 (assuming no exercise of the over-allotment option). |
The as adjusted information below is illustrative only and our capitalization following the completion of this offering is subject to adjustment based on the public offering price of our common stock and other terms of this offering determined at pricing. You should read this table together with our financial statements and the related notes included elsewhere in this prospectus and the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
|
|
| June 30, 2022 |
|
|||||
|
|
| Actual |
|
| As Adjusted |
|
||
| Cash and cash equivalents |
| $ | 8,351,945 |
|
| $ | 14,698,945 |
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
|
| Series C convertible preferred stock, $0.001 par value, 1,785,715 shares authorized; 1,785,715 shares issued and outstanding, actual and as adjusted |
|
| 1,790 |
|
|
| 1,790 |
|
| Series D convertible preferred stock, $0.001 par value, 1,016,014 shares authorized; 1,016,004 shares issued and outstanding, actual and as adjusted |
|
| 1,015 |
|
|
| 1,015 |
|
| Common stock, $0.001 par value, 200,000,000 shares authorized; 43,802,147 shares issued and outstanding, actual; 47,449,281 shares issued and outstanding, as adjusted |
|
| 43,803 |
|
|
| 47,449 |
|
| Additional paid-in capital |
|
| 100,699,797 |
|
|
| 107,043,151 |
|
| Accumulated deficit |
|
| (95,827,137 | ) |
|
| (95,827,137 | ) |
| Total stockholder’ equity |
|
| 4,919,268 |
|
|
| 11,266,268 |
|
| Total capitalization |
| $ | 4,919,268 |
|
| $ | 11,266,268 |
|
If the underwriters exercise the over-allotment option in full, each of our as adjusted cash, total stockholders’ equity and total capitalization would be $15,692,545, $12,259,868 and $12,259,868, respectively.
The table above excludes the following shares:
|
| · | 20,927,370 shares of our common stock issuable upon the exercise of options which we granted to our officers, directors, and employees under the 2021 Plan (as defined below) at a weighted average exercise price of $1.628 per share (includes 13,976,370 shares of common stock issuable upon the exercise of options originally granted under our 2011 Plan (as defined below) at a weighted average exercise price of $1.483 per share, and now subsumed under our 2021 Plan); |
|
|
|
|
|
| · | 15,722,750 additional shares of our common stock that are reserved for issuance under the 2021 Plan; |
|
|
|
|
|
| · | 8,108,356 shares of our common stock issuable upon the conversion of our series C convertible preferred stock and series D convertible preferred stock; |
|
|
|
|
|
| · | 9,020,264 shares of our common stock issuable upon the conversion of convertible debentures; |
|
|
|
|
|
| · | 21,651,513 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $1.003 per share; and |
|
|
|
|
|
| · | up to 289,800 shares of our common stock issuable upon exercise of the representative’s warrants issued in connection with this offering. |
| 24 |
| Table of Contents |
Dilution in net tangible book value per share to new investors in our common stock is the amount by which the offering price paid by the purchasers of the shares of our common stock sold in this offering exceeds the pro forma net tangible book value per share of common stock immediately after this offering. Net tangible book value per share is determined at any date by subtracting our total liabilities from the total book value of our tangible assets and dividing the difference by the number of shares of common stock deemed to be outstanding at that date.
The net tangible book value of our common stock as of June 30, 2022 was approximately $4,919,268, or approximately $0.11 per share.
Pro forma as adjusted net tangible book value dilution per share to new investors represents the difference between the amount per share paid by purchasers of our common stock in this offering and the pro forma as adjusted net tangible book value per share of our common stock immediately after completion of this offering. Investors participating in this offering will incur immediate, substantial dilution. After giving effect to the issuance of 47,134 shares of common stock subsequent to June 30, 2022 and our sale of 3,600,000 shares of our common stock in this offering at a public offering price of $2.00 per share, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses, our pro forma as adjusted net tangible book value as of June 30, 2022 would have been approximately $11,266,268, or approximately $0.24 per share. This amount represents an immediate increase in pro forma net tangible book value of $0.13 per share to existing stockholders and an immediate dilution in pro forma net tangible book value of $1.76 per share to purchasers of our common stock in this offering, as illustrated in the following table.
| Public offering price per share |
|
|
|
| $ | 2.00 |
|
|
| Historical net tangible book value per share as of June 30, 2022 |
| $ | 0.11 |
|
|
|
|
|
| Increase in pro forma as adjusted net tangible book value per share attributable to new investors purchasing shares in this offering |
| $ | 0.13 |
|
|
|
|
|
| Pro forma as adjusted net tangible book value per share after this offering |
|
|
|
|
| $ | 0.24 |
|
| Dilution per share to new investors purchasing shares in this offering |
|
|
|
|
| $ | 1.76 |
|
If the underwriters exercise the over-allotment option in full, the pro forma as adjusted net tangible book value per share of our common stock, as adjusted to give effect to this offering, would be $0.26 per share, and the dilution in pro forma net tangible book value per share to new investors purchasing shares of our common stock in this offering would be $1.74 per share.
To the extent that any outstanding options are exercised, new options, restricted stock units or other securities are issued under our stock-based compensation plans, or new shares of preferred stock are issued, or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering.
| 25 |
| Table of Contents |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis summarizes the significant factors affecting our operating results, financial condition, liquidity and cash flows of our company as of and for the periods presented below. The following discussion and analysis should be read in conjunction with our financial statements and the related notes thereto included elsewhere in this prospectus. The discussion contains forward-looking statements that are based on the beliefs of management, as well as assumptions made by, and information currently available to, our management. Actual results could differ materially from those discussed in or implied by forward-looking statements as a result of various factors, including those discussed below and elsewhere in this prospectus, particularly in the sections titled “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements.”
Overview
We are focused on the development and commercialization of proprietary biosensor technologies which, when paired with our artificial intelligence, or AI, deep learning platform, are capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify and measure the unique “signature” of said materials or analytes. We call these our “Bio-RFID™” technology platform when pertaining to radio and microwave spectroscopy; and “ChromaID” technology platform when pertaining to optical spectroscopy. The data obtained with our biosensor technology is analyzed with our trade secret algorithms which are driven by our AI deep learning platform.
ChromaID is the first technology developed and patented by our company. For the past several years, we have focused on extensions and new patentable inventions that are derived from and extend beyond our ChromaID technology and intellectual property. We call this technology platform Bio-RFID. The rapid advances made with our Bio-RFID technology in our laboratory have caused us to move quickly into the commercialization phase of our company as we work to create revenue generating products for the marketplace. Today, the primary focus of our company is on our Bio-RFID technology, its commercialization and development of related patent assets. Through our wholly owned subsidiaries, our company works to exploit additional opportunities and markets that our broad intellectual property and trade secret portfolio addresses.
On April 30, 2020, we incorporated our wholly owned subsidiary, Particle, Inc. Particle is focused on the development and commercialization of our extensive intellectual property relating to electromagnetic energy outside of the medical diagnostic arena which remains our company’s singular focus. Since incorporation, Particle has engaged in research and development activities on threaded light bulbs that have a warm white light and can inactivate germs, including bacteria and viruses. Particle is now looking for partners to take this product to market.
On September 17, 2021 we incorporated our wholly owned subsidiary, AI Mind, Inc., for the purpose of identifying and capitalizing on market opportunities for our AI deep learning platform (discussed below). The first activity undertaken by AI Mind was the creation of graphical images expressed as non-fungible tokens, or NFTs, utilizing the AI deep learning platform. During the nine months ended June 30, 2022, AI Mind, operating our AI deep learning platform, began generating revenue from digital asset sales of NFT’s and had sales of $4,360,000.
Recent Developments
On May 24, 2022, we announced the appointment of the following new officers: Peter Conley, as Chief Financial Officer and Senior Vice President of Intellectual Property, Steven Kent, as Chief Product Officer, and Leonardo Troutwein, who joined our company in February 2021, as Chief Marketing Officer.
Impact of COVID-19 Pandemic
Over the past two years the impact of COVID-19 has had adverse effects on our business by slowing down our ability to work with third parties outside of Seattle on testing and validation. We have witnessed supply chain related delays and increasing costs due to inflation. It is difficult to predict what other adverse effects, if any, COVID-19 and related matters can have on our business, or against the various aspects of same.
On January 30, 2020, the World Health Organization (“WHO”) announced a global health emergency caused by a new strain of the coronavirus (“COVID-19”) and advised of the risks to the international community as the virus spread globally. In March 2020, the WHO classified the COVID-19 outbreak as a pandemic based on the rapid increase in exposure globally. The spread of COVID-19 coronavirus caused public health officials to recommend precautions to mitigate the spread of the virus, especially as to travel and congregating in large numbers. Over time, the incidence of COVID-19 and its variants has diminished although periodic spikes in incidence occur. Consequently, restrictions imposed by various governmental health organizations may change over time. Several states have lifted restrictions only to reimpose such restrictions as the number of cases rise and new variants arise.
| 26 |
| Table of Contents |
It is difficult to isolate the impact of the pandemic on our business, results of operations, financial condition and our future strategic plans.
The Company may experience long-term disruptions to its operations resulting from changes in government policy or guidance; quarantines of employees, customers and suppliers in areas affected by the pandemic and the presence of new variants of COVID-19; and closures of businesses or manufacturing facilities critical to its business or supply chains. The Company is actively monitoring, and will continue to actively monitor, the pandemic and the potential impact on its operations, financial condition, liquidity, suppliers, industry and workforce.
Principal Factors Affecting Our Financial Performance
Our operating results are primarily affected by the following factors:
|
| · | The ability of our research and development team to produce an FDA clearance quality technology; |
|
|
|
|
|
| · | Our ability to recruit and maintain quality personnel with the talent to bring our technology to the market; |
|
|
|
|
|
| · | The production of market ready products which can sustain FDA clearance quality results; |
|
|
|
|
|
| · | The clearance by the FDA after their rigorous clinical trial process of our products for the marketplace; |
|
|
|
|
|
| · | The receptivity of the marketplace and the addressable diabetes community to our new non-invasive glucose monitoring technology’ and |
|
|
|
|
|
| · | Access to sufficient capital to support the Company until its products achieve FDA clearance and are accepted in the marketplace. |
Results of Operations
Comparison of Nine Months Ended June 30, 2022 and 2021
The following table presents certain consolidated statement of operations information and presentation of that data as a percentage of change from period-to-period.
| (dollars in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
| Nine Months Ended June 30, |
|
|||||||||||||
|
|
| 2022 |
|
| 2021 |
|
| $ Variance |
|
| % Variance |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| Revenue- Digital Asset Sales |
| $ | 4,360 |
|
| $ | - |
|
| $ | 4,360 |
|
|
| 100.0 | % |
| Research and Development and Operating Expenses- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development expenses |
|
| 3,407 |
|
|
| 3,094 |
|
|
| 313 |
|
|
| -10.1 | % |
| Selling, general and administrative expenses |
|
| 4,255 |
|
|
| 4,934 |
|
|
| (679 | ) |
|
| 13.8 | % |
| Selling and transactional costs for digital assets |
|
| 3,437 |
|
|
| - |
|
|
| 3,437 |
|
|
| -100.0 | % |
| Total research and development and operating expenses |
|
| 11,099 |
|
|
| 8,028 |
|
|
| 3,071 |
|
|
| -38.3 | % |
| Operating loss |
|
| (6,739 | ) |
|
| (8,028 | ) |
|
| 1,289 |
|
|
| 16.1 | % |
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
|
| (8,024 | ) |
|
| (9,736 | ) |
|
| 1,712 |
|
|
| 17.6 | % |
| Other income |
|
| 262 |
|
|
| - |
|
|
| 262 |
|
|
| 100.0 | % |
| Total other (expense), net |
|
| (7,762 | ) |
|
| (9,736 | ) |
|
| 1,974 |
|
|
| 20.3 | % |
| Loss before income taxes |
|
| (14,501 | ) |
|
| (17,764 | ) |
|
| 3,263 |
|
|
| 18.4 | % |
| Income tax expense |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 0.0 | % |
| Net loss |
| $ | (14,501 | ) |
| $ | (17,764 | ) |
| $ | 3,263 |
|
|
| 18.4 | % |
Revenues. Revenue- digital asset sales for the nine months ended June 30, 2022 was $4,360,000 as compared to $0 for the nine months ended June 30, 2021. Our Artificial Intelligence (AI) deep learning platform has generated revenue- digital asset sales of $4,360,000 from Non-Fungible Token (NFT) sales.
| 27 |
| Table of Contents |
Research and Development Expenses. Research and development expenses for the nine months ended June 30, 2022 increased $313,000 to $3,407,000 as compared to $3,094,000 for the nine months ended June 30, 2021. The increase was due increased personnel, use of consultant and expenditures related to the development of our Bio-RFID™ technology.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the nine months ended June 30, 2022 decreased $679,000 to $4,255,000 as compared to $4,934,000 for the nine months ended June 30, 2021. The decrease primarily was due to (i) a decrease of $1,209,000 in stock based compensation; offset by (ii) $431,000 in increased marketing expenses; and (iii) other increases of $99,000. As part of the selling, general and administrative expenses for the nine months ended June 30, 2022 and 2021, we recorded $279,000 and $424,000, respectively, of investor relationship expenses and business development expenses.
Selling and Transactional Costs for Digital Asset Sales. Selling and transactional cots for digital asset sales were $3,437,000 for the nine months ended June 30, 2022. Our Artificial Intelligence (AI) deep learning platform has generated revenue- digital asset sales of $4,360,000 from Non-Fungible Token (NFT) sales. Such costs included digital asset conversion loss, consulting, bonus compensation transaction fees, taxes, royalties and other costs.
Other (Expense), Net. Other expense, net for the nine months ended June 30, 2022 was $7,762,000 as compared to other expense, net of $9,736,000 for the nine months ended June 30, 2021. The other expense, net for the nine months ended June 30, 2022 included (i) interest expense of $8,045,000 related to convertible notes payable and the amortization of the beneficial conversion feature and value of warrants issued; and offset by (ii) other income of $262,000 primarily related to the forgiveness of notes payable- PPP loans . The other expense, net for the nine months ended June 30, 2021 included interest expense of $9,736,000 related to convertible notes payable and the amortization of the beneficial conversion feature.
Net Loss. Net loss for the nine months ended June 30, 2022 was $14,501,000 as compared to $17,764,000 for the nine months ended June 30, 2021. The net loss for the nine months ended June 30, 2022 included non-cash expenses of $9,243,000. The non-cash items include (i) depreciation and amortization of $219,000; (ii) issuance of common stock for services and expenses of $153,000; (iii) issuance of common stock warrants for service of $71,000; (iv) stock based compensation- stock options of $1,556,000; (v) interest expense for warrant modification of $244,000; (vi) gain on forgiveness of note payable- PPP loans of $253,000; (vii) amortization of debt discount as interest expense of $7,273,000; and offset by (viii) other of $20,000.
The net loss for the nine months ended June 30, 2021 included non-cash expenses of $12,203,000. The non-cash items include (i) depreciation and amortization of $166,000; (ii) issuance for capital stock for services and expenses of $203,000; (iii) stock based compensation- warrants of $2,194,000; (iv) stock based compensation- stock options of $571,000; (v) amortization of debt discount as interest expense of $9,071,000; and offset by (vi) other of $2,000.
Segment Reporting
Our management considers our business to currently consist of three operating segments (i) the development of the Bio-RFID™” and “ChromaID” technologies; (ii) Particle, Inc. technology; and (iii) AI sales of NFT products. Particle commenced operations in the three months ended June 30, 2020. AI commenced operations during the nine months ended June 30, 2022. For a reporting of the operating results for these three segments for the three and nine month periods ended June 30, 2022, see Note 12 to our unaudited consolidated financial statements of the three and nine months ended June 30, 2022.
| 28 |
| Table of Contents |
Comparison of Years Ended September 30, 2021 and 2020
The following table presents certain consolidated statement of operations information and presentation of that data as a percentage of change from year-to-year.
(dollars in thousands)
|
|
| Years Ended September 30, |
|
|||||||||||||
|
|
| 2021 |
|
| 2020 |
|
| $ Variance |
|
| % Variance |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| Revenue |
| $ | - |
|
| $ | 122 |
|
| $ | (122 | ) |
|
| -100.0 | % |
| Cost of sales |
|
| - |
|
|
| 70 |
|
|
| (70 | ) |
|
| 100.0 | % |
| Gross profit |
|
| - |
|
|
| 52 |
|
|
| (52 | ) |
|
| -100.0 | % |
| Research and development expenses |
|
| 3,970 |
|
|
| 2,034 |
|
|
| 1,936 |
|
|
| -95.2 | % |
| Selling, general and administrative expenses |
|
| 6,476 |
|
|
| 4,844 |
|
|
| 1,632 |
|
|
| -33.7 | % |
| Operating loss |
|
| (10,446 | ) |
|
| (6,826 | ) |
|
| (3,620 | ) |
|
| -28.9 | % |
| Other (expense) income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
|
| (14,914 | ) |
|
| (6,094 | ) |
|
| (8,820 | ) |
|
| -144.7 | % |
| Other income |
|
| - |
|
|
| 65 |
|
|
| (65 | ) |
|
| -100.0 | % |
| (Loss) gain on debt settlements |
|
| - |
|
|
| (708 | ) |
|
| 708 |
|
|
| 100.0 | % |
| Total other income (expense) |
|
| (14,914 | ) |
|
| (6,737 | ) |
|
| (8,177 | ) |
|
| -121.4 | % |
| (Loss) before income taxes |
|
| (25,360 | ) |
|
| (13,563 | ) |
|
| (11,797 | ) |
|
| -87.0 | % |
| Income taxes - current (benefit) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 0.0 | % |
| Net (loss) |
| $ | (25,360 | ) |
| $ | (13,563 | ) |
| $ | (11,797 | ) |
|
| -87.0 | % |
Sales. Revenue for the year ended September 30, 2021 decreased $122,000 to $0 as compared to $122,000 for the year ended September 30, 2020. TransTech, Inc., a subsidiary which was closed on September 30, 2020.
Cost of Sales. Cost of sales for the year ended September 30, 2021 decreased $70,000 to $0 as compared to $70,000 for the year ended September 30, 2020. TransTech closed September 30, 2020.
Research and Development Expenses. Research and development expenses for the year ended September 30, 2021 increased $1,936,000 to $3,970,000 as compared to $2,034,000 for the year ended September 30, 2020. The increase was due to increased personnel, use of consultant and expenditures related to the development of our Bio-RFID™ and Particle technologies.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the year ended September 30, 2021 increased $1,632,000 to $6,476,000 as compared to $4,844,000 for the year ended September 30, 2020. The increase primarily was primarily due to increased stock based compensation of $1,873,000. On December 15, 2020, we issued a warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is exercisable at $1.53 per share and was valued using a “Black-Scholes” model at $1,812,000. As part of the selling, general and administrative expenses for the years ended September 30, 2021 and 2020, we recorded $613,000 and $206,000, respectively, of investor relationship expenses and business development expenses.
Other (Expense), Net. Other expense, net for the year ended September 30, 2021 was $14,914,000 as compared to other expense, net of $6,737,000 for the year ended September 30, 2020. The other expense, net for the year ended September 30, 2021 included the interest expense related to convertible notes payable and the amortization of the beneficial conversion feature and value of warrants issued. During the year ended September 30, 2020, we closed a private placement and received gross proceeds of $14,914,000 in exchange for issuing Subordinated Convertible Notes and Warrants in a private placement to accredited investors, pursuant to a series of substantially identical Securities Purchase Agreements, common stock warrants, and related documents.
Net Loss. Net loss for the year ended September 30, 2021 was $25,360,000 as compared to $13,563,000 for the year ended September 30, 2020. The net loss for the year ended September 30, 2021 included non-cash expenses of $17,701,000. The non-cash items include (i) depreciation and amortization of $201,000; (ii) issuance for capital stock for services and expenses of $203,000; (iii) stock-based compensation- warrants of $2,547,000; (iv) stock based compensation- stock options of $1,029,000; (v) amortization of debt discount as interest expense of $13,722,000; and offset by (vi) other of $1,000. On December 15, 2020, we issued a warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is convertible at $1.53 per share and was valued using a “Black-Scholes” model at $1,812,000.
The net loss for the year ended September 30, 2020 included non-cash items of non-cash expenses of $9,366,000. The non-cash items include (i) depreciation and amortization of $243,000; (ii) issuance of capital stock for services and expenses of $1,045,000; (iii) stock based compensation of $1,702,000; (iv) amortization of debt discount as interest expense of $5,663,000; (v) loss on debt settlement of $825,000; and (vi) other of $5,000, offset by (vii) gain on debt settlement of $117,000.
| 29 |
| Table of Contents |
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by operations, levels of accounts receivable and accounts payable and capital expenditures.
As of June 30, 2022, we had cash and cash equivalents of approximately $8,352,000 and net working capital of approximately $6,042,000 (exclusive of convertible notes payable). We have experienced net losses since inception. As of June 30, 2022, we had an accumulated deficit of $95,827,000 and net losses in the amount of $14,501,000, $25,360,000, and $13,563,000 for the nine months ended June 30, 2022 and the years ended September 30, 2021 and 2020, respectively. We incurred non-cash expenses of $9,243,000, $17,701,000 and $9,366,000 during the nine months ended June 30, 2022 and the years ended September 30, 2021 and 2020, respectively.
On March 15, 2021, we closed private placement for gross proceeds of $14,209,000 in exchange for issuing subordinated convertible notes and warrants to purchase 3,552,250 shares of our common stock in a private placement to accredited investors. These convertible notes were automatically converted into shares of our common stock at a conversion price of $2.00 per share starting on March 9, 2022. The convertible notes had an original principal amount of $14,209,000 with an annual interest of 8%. Both the principal amount and the interest were payable on a payment-in-kind basis in shares of our common stock.
We believe that our cash on hand will be sufficient to fund our operations through June 30, 2023.
We have financed our corporate operations and our technology development through the issuance of convertible debentures, the issuance of preferred stock, the sale of common stock and the exercise of warrants.
On July 29, 2022, we filed a Form S-1 registration statement, of which this prospectus forms a part, for this new offering of 3 million shares of our common stock with a public offering price of $2.00 per share.
The proceeds of warrants currently outstanding, which are not expected to be exercised on a cashless basis, may generate potential proceeds of up to $16,384,000. We cannot provide assurance that any of these warrants will be exercised.
Cash Flow
The following table provides detailed information about our net cash flow for the period indicated:
|
|
| Nine Months Ended June 30, |
|
| Years Ended September 30, |
|
||||||||||
|
|
| 2022 |
|
| 2021 |
|
| 2021 |
|
| 2020 |
|
||||
| Net cash used in operating activities |
| $ | (3,691,286 | ) |
| $ | (5,144.506 | ) |
| $ | (6,850,699 | ) |
| $ | (3,913,803 | ) |
| Net cash used in investing activities |
|
| (843,557 | ) |
|
| (47,553 | ) |
|
| (299,525 | ) |
|
| (70,134 | ) |
| Net cash provided by financing activities |
|
| 628,570 |
|
|
| 14,764,063 |
|
|
| 15,110,263 |
|
|
| 6,381,280 |
|
| Net (decrease) increase in cash and cash equivalents |
|
| (3,906,273 | ) |
|
| 9,572,004 |
|
|
| 7,960,039 |
|
|
| 2,397,343 |
|
| Cash and cash equivalents at beginning of period |
|
| 12,258,218 |
|
|
| 4,298,179 |
|
|
| 4,298,179 |
|
|
| 1,900,836 |
|
| Cash and cash equivalent at end of period |
| $ | 8,351,945 |
|
| $ | 13,870,183 |
|
| $ | 12,258,218 |
|
| $ | 4,298,179 |
|
Net cash used in operating activities for the nine months ended June 30, 2022 and 2021 was $3,691,000 and $5,145,000, respectively. The net cash used in operating activities for the nine months ended June 30, 2022 was primarily related to (i) a net loss of $14,501,000; offset by (ii) working capital changes of $1,567,000 related to Our Artificial Intelligence (AI) Deep Learning Platform has generated initial revenue from Non-Fungible Token (NFT) sales and incurred certain expenses; and (iii) non-cash expenses of $9,243,000. The non-cash items include (iv) depreciation and amortization of $219,000; (v) issuance of common stock for services and expenses of $153,000; (vi) issuance of common stock warrants for service of $71,000; (vii) stock based compensation- stock options of $1,556,000; (viii) interest expense for warrant modification of $244,000; (ix) gain on forgiveness of note payable- PPP loans of $253,000; (x) amortization of debt discount as interest expense of $7,273,000; and offset by (xi) other of $20,000.
| 30 |
| Table of Contents |
Net cash used in operating activities for the nine months ended June 30, 2021 was $5,145,000. This amount was primarily related to (i) a net loss of $17,764,000; offset by (ii) working capital changes of $416,000; and (iii) non-cash expenses of $12,203,000. The non-cash items include (iv) depreciation and amortization of $166,000; (v) issuance for capital stock for services and expenses of $203,000; (vi) stock based compensation- warrants of $2,194,000; (vii) stock based compensation- stock options of $571,000; (viii) amortization of debt discount as interest expense of $9,071,000; and offset by (ix) other of $2,000.
Net cash used in operating activities for the years ended September 30, 2021 and 2020 was $6,851,000 and $3,914,000, respectively. The net cash used in operating activities for the year ended September 30, 2021 was primarily related to (i) a net loss of $25,360,000; offset by (ii) working capital changes of $810,000; and (ii) non-cash expenses of $13,050,000. The non-cash items include (iii) depreciation and amortization of $201,000; (iv) issuance for capital stock for services and expenses of $203,000; (v) stock based compensation- warrants of $2,547,000; (vi) stock based compensation- stock options of $1,029,000; (vii) amortization of debt discount as interest expense of $13,722,000; and offset by (viii) other of $1,000. On December 15, 2020, we issued a warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is convertible at $1.53 per share and was valued using a “Black-Scholes” model at $1,812,000. The net cash used in operating activities for the year ended September 30, 2020 was primarily related to (i) a net loss of $13,563,000; offset by (ii) working capital changes of $283,000; and (ii) non-cash expenses of $9,366,000. The non-cash items include (iii) depreciation and amortization of $243,000; (iv) issuance for capital stock for services and expenses of $1,405,000; (v) stock based compensation- warrants of $1,702,000; (vi) amortization of debt discount as interest expense of $5,663,000; (vii) loss on debt settlement of $825,000; (viii) other of $5,000 and offset by (ix) gain on debt settlement of $117,000.
Net cash used in investing activities for the nine months ended June 30, 2022 and 2021 was $844,000 and $48,000, respectively. There amounts were primarily related to the investment in equipment for research and development, and was $300,000 and $70,000 for the years ended September 30, 2021 and 2020, respectively. These amounts were primarily related to the investment in equipment for research and development.
Net cash provided by financing activities for the nine months ended June 30, 2022 and 2021 was $629,000 and $14,764,000, respectively. The net cash provided by financing activities for the nine months ended June 30, 2022 was primarily related to (i) proceeds from the issuance of common stock for the exercise of warrants of $794,000; (ii) proceeds from the issuance of common stock for the exercise of stock option grants of $14,000; and offset by the settlement of notes payable- PPP loans. Net cash provided by financing activities for the nine months ended June 30, 2021 was $14,764,000. This amount was primarily related to (i) issuance of Simple Agreements for future Equity of $340,000; (ii) $14,209,000 related to proceeds from convertible notes payable; (iii) proceeds from notes payable- PPP of $206,000; and (iv) proceed from the issuance of common stock for the exercise of warrants of $653,000; and offset by (v) payment of issuance costs from notes payable of $727,000.
Net cash provided by financing activities for the years ended September 30, 2021 and 2020 was $15,110,000 and $6,381,000, respectively. The net cash provided by financing activities for the year ended September 30, 2021 was primarily related to (i) issuance of Simple Agreements for future Equity of $340,000; (ii) $14,209,000 related to proceeds from convertible notes payable; (iii) proceeds from notes payable- PPP of $206,000; (iv) proceeds from the issuance of common stock for the exercise of warrants of $1,313,000; (v) proceeds from the issuance of common stock for the exercise of stock option grants of $23,000; and offset by (vi) payment of issuance costs from notes payable of $727,000 and (vii) repayments on Simple Agreements for Future Equity.
Private Placement
On March 15, 2021, we closed a private placement for gross proceeds of $14,209,000 in exchange for issuing subordinated convertible notes and warrants to purchase 3,552,250 shares of our common stock in a private placement to accredited investors. These convertible notes were automatically converted into shares of our common stock at a conversion price of $2.00 per share starting on March 9, 2022. The Convertible Notes had an original principal amount of $14,209,000 with an annual interest of 8%. Both the principal amount and the interest were payable on a payment-in-kind basis in shares of our common stock
| 31 |
| Table of Contents |
Contractual Obligations
Our contractual cash obligations as of June 30, 2022 are summarized in the table below:
|
|
|
|
| Less Than |
|
|
|
|
|
| Greater Than |
|
||||||||
| Contractual Cash Obligations (1) |
| Total |
|
| 1 Year |
|
| 1-3 Years |
|
| 3-5 Years |
|
| 5 Years |
|
|||||
| Operating leases |
| $ | 271,816 |
|
| $ | 147,903 |
|
| $ | 123,913 |
|
| $ | - |
|
| $ | - |
|
| Convertible notes payable |
|
| 2,255,066 |
|
|
| 2,255,066 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
| $ | 2,526,882 |
|
| $ | 2,402,969 |
|
| $ | 123,913 |
|
| $ | - |
|
| $ | - |
|
|
| (1) | Convertible notes payable includes $2,255,066 that can be converted into common stock upon demand. We expect to incur capital expenditures related to the development of the “Bio-RFID™” and “ChromaID” technologies. None of the expenditures are contractual obligations as of June 30, 2022. |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements (as that term is defined in Item 303 of Regulation S-K) that are reasonably likely to have a current or future material effect on our financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies
The preparation of financial statements in conformity with GAAP requires our management to make assumptions, estimates and judgments that affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial condition and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments. We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements:
Revenue Recognition. We determine revenue recognition from contracts with customers through the following steps:
|
| · | identification of the contract, or contracts, with the customer; |
|
|
|
|
|
| · | identification of the performance obligations in the contract; |
|
|
|
|
|
| · | determination of the transaction price; |
|
|
|
|
|
| · | allocation of the transaction price to the performance obligations in the contract; and |
|
|
|
|
|
| · | recognition of the revenue when, or as, our company satisfies a performance obligation. |
Revenue is recognized when control of the promised goods or services is transferred to the customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. During the nine months ended June 30, 2022, our artificial intelligence (AI) deep learning platform began generating revenue from digital asset sales of NFT’s. Our engineering team, using its research date, AI and proprietary algorithms, produced NFT’s in the form of digital art. The NFT’s produced had no recorded cost basis.
Digital Asset Sales. Revenue includes sale of NFT’s in the form of digital art generated from our artificial intelligence deep learning platform. We use the NFT exchange, OpenSea, to facilitate the transaction with the customer. Through OpenSea, we have custody and control of the NFT prior to the delivery to the customer and record revenue at the point in time when the NFT is delivered to the customer and the customer pays. We have no obligations for returns, refunds or warranty after the NFT sale. The customer pays in the form of transferring the crypto currency digital asset, Ethereum. The value of the sale is determined based on the value of the Ethereum crypto currency received as consideration. Payment is required before the NFT is delivered. Each NFT that is generated produces a unique identifying code. We also earn a royalty of up to 10%, when an NFT is resold by its owner in a secondary market transaction. We recognize this royalty as revenue when the transaction is consummated, and they receive compensation.
After the sale of the NFT, the Ethereum is converted to US dollars as soon as practically possible. We record the total value of the gross NFT sale in revenue. Costs incurred in connection with the NFT transaction are recorded in the statement of operations as Selling and Transactional Cost of Digital Assets and include costs to outside consultants, estimated employee and CEO special bonus compensation, and estimated sales and use tax.
| 32 |
| Table of Contents |
Research and Development Expenses. Research and development expenses consist of the cost of employees, consultants and contractors who design, engineer and develop new products and processes as well as materials, supplies and facilities used in producing prototypes. Our current research and development efforts are primarily focused on improving our Bio-RFID technology, extending its capacity and developing new and unique applications for this technology. As part of this effort, we conduct on-going laboratory testing to ensure that application methods are compatible with the end-user and regulatory requirements, and that they can be implemented in a cost-effective manner. We also are actively involved in identifying new applications. Our current internal team along with outside consultants has considerable experience working with the application of our technologies and their applications. We engage third party experts as required to supplement our internal team. We believe that continued development of new and enhanced technologies is essential to our future success. We incurred expenses of $3,406,996, $3,969,972 and $2,033,726 for the nine months ended June 30, 2022 and the years ended September 30, 2021 and 2020, respectively, on development activities.
Equipment. Equipment consists of machinery, leasehold improvements, furniture and fixtures and software, which are stated at cost less accumulated depreciation and amortization. Depreciation is computed by the straight-line method over the estimated useful lives or lease period of the relevant asset, generally 2-5 years, except for leasehold improvements which are depreciated over 5 years.
Fair Value Measurements and Financial Instruments. ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
Level 1 – Quoted prices in active markets for identical assets and liabilities;
Level 2 – Inputs other than level one inputs that are either directly or indirectly observable; and.
Level 3 - Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The recorded value of other financial assets and liabilities, which consist primarily of cash and cash equivalents, accounts receivable, other current assets, and accounts payable and accrued expenses approximate the fair value of the respective assets and liabilities as of June 30, 2022 and September 30, 2021 are based upon the short-term nature of the assets and liabilities.
We have a money market account which is considered a level 1 asset. The balance as of June 30, 2022 and September 30, 2021 was $6,041,016 and $12,217,714, respectively.
Derivative Financial Instruments. Pursuant to ASC 815 “Derivatives and Hedging”, we evaluate all of our financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. We then determine if embedded derivative must be bifurcated and separately accounted for. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. For stock-based derivative financial instruments, we use a Black-Scholes-Merton option pricing model to value the derivative instruments at inception and on subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within twelve months of the balance sheet date. We determined that the conversion features for purposes of bifurcation within convertible notes payable were immaterial and there was no derivative liability to be recorded as of June 30, 2022 and September 30, 2021.
Stock Based Compensation. We have share-based compensation plans under which employees, consultants, suppliers and directors may be granted restricted stock, as well as options and warrants to purchase shares of common stock at the fair market value at the time of grant. Stock-based compensation cost to employees is measured by us at the grant date, based on the fair value of the award, over the requisite service period under ASC 718. For options issued to employees, we recognize stock compensation costs utilizing the fair value methodology over the related period of benefit.
Convertible Securities. Based upon ASC 815-15, we have adopted a sequencing approach regarding the application of ASC 815-40 to convertible securities. We will evaluate our contracts based upon the earliest issuance date. In the event partial reclassification of contracts subject to ASC 815-40-25 is necessary, due to our inability to demonstrate we have sufficient shares authorized and unissued, shares will be allocated on the basis of issuance date, with the earliest issuance date receiving first allocation of shares. If a reclassification of an instrument were required, it would result in the instrument issued latest being reclassified first.
| 33 |
| Table of Contents |
Overview
We are focused on the development and commercialization of proprietary biosensor technologies which, when paired with our AI deep learning platform, are capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify and measure the unique “signature” of said materials or analytes. We call this our “Bio-RFID™” technology platform, when pertaining to radio and microwave spectroscopy, and our “ChromaID” technology platform, when pertaining to optical spectroscopy. The data obtained with our biosensor technology is analyzed with our trade secret algorithms which are driven by our AI deep learning platform.
ChromaID is the first technology developed and patented by our company. For the past several years, we have focused on extensions and new patentable inventions that are derived from and extend beyond our ChromaID technology and intellectual property. We call this technology platform Bio-RFID. The rapid advances made with our Bio-RFID technology in our laboratory have caused us to move quickly into the commercialization phase of our Company as we work to create revenue generating products for the marketplace. Today, the primary focus of our company is on our Bio-RFID technology and our commercialization and development of related patent assets. Through our wholly owned subsidiaries, our company works to exploit additional opportunities and markets that our broad intellectual property and trade secret portfolio addresses.
Corporate History and Structure
Know Labs, Inc. was incorporated under the laws of the State of Nevada in 1998. Since 2007, our Company has been focused primarily on research and development of proprietary spectroscopic technologies spanning the electromagnetic spectrum.
On April 30, 2020, we incorporated Particle, Inc., or Particle, as a wholly-owned subsidiary in the State of Nevada. Particle is focused on the development and commercialization of our extensive intellectual property relating to electromagnetic energy outside of the medical diagnostic arena which remains the parent company’s singular focus.
On September 17, 2021, we incorporated of AI Mind, Inc., or AI Mind, as a wholly-owned subsidiary in the State of Nevada. AI Mind is focused on monetizing the AI deep learning platform.
The Know Labs Technology
We have internally and under contract with third parties developed proprietary platform technologies to uniquely identify and measure almost any organic and inorganic material or analyte. Our patented technology utilizes electromagnetic energy along a wide range of the electromagnetic spectrum from visible light and infrared to radio and microwave wavelengths to perform analytics which allow the user to accurately identify and measure materials and analytes depending upon the specified targets or endpoints and field of use. Our company’s proprietary platform technologies are called Bio-RFID and ChromaID.
Our most recent technology platform is called Bio-RFID, which utilizes spectroscopy at higher wavelengths than ChromaID’s optical range, to span radio wave and microwave segments of the electromagnetic spectrum. Working in our lab over the last three years, we have developed extensions and new inventions derived in part from our ChromaID technology which we refer to as Bio-RFID. We believe an important competitive differentiator for Bio-RFID to be its ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so concurrently, and in real time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring. We are rapidly advancing the development of this technology by increasing its accuracy, sensitivity, and specificity. We have announced detailed results confirming that we have successfully been able to non-invasively measure blood glucose levels in humans.
The ability of our company to obtain exacting results from the data obtained from our Bio-RFID sensor technology, also referred to as Radio Frequency Spectroscopy or RF Spectroscopy is a consequence of the application of our company’s trade secret algorithms. Our company has worked for the last several years on the AI and machine learning, or ML, that drives the accurate pattern recognition of our algorithms. This work has led to the development of a robust AI deep learning platform. This AI deep learning platform drives the data pattern recognition for Bio-RFID’s exacting determination of blood glucose levels. It can also provide the data recognition for blood alcohol and blood oxygen levels which our company has also identified in preliminary tests. It will provide the analytics for the long list of other analytes in the human body that our company will pursue non-invasive detection of, many of which are set forth in our company’s issued patent USPTO 11,033,208 B1. Our company’s AI deep learning platform will be monetized through our subsidiary AI Mind.
| 34 |
| Table of Contents |
We continue to build the internal and external development team necessary to commercialize this newly discovered technology as well as make additional patent filings covering the intellectual property created with these new inventions. The first applications of our Bio-RFID technology will be in a product marketed as a glucose monitor. It will provide the user with real time information on their blood glucose levels. This product will require US Food and Drug Administration, or FDA, clearance prior to its introduction to the market, which we plan to pursue. We have previously announced two versions of our non-invasive glucose monitoring device. We have identified these as the KnowU™ and the UBand™. The KnowU will be a desk top version with a portable monitoring device for periodic glucose monitoring and the UBand will be a wearable for continuous glucose monitoring.
We have also announced the results of laboratory-based comparison testing between our Bio-RFID technology and the leading continuous glucose monitors from Abbott Labs (Freestyle Libre®) and DexCom (G6®). These results provide evidence of a high degree of correlation between our Bio-RFID technology and the current industry leaders and their continuous glucose monitors. Our patented technology is fundamentally differentiated from these industry leaders as our technology completely non-invasively monitors blood glucose levels.
We have begun the internal process to pursue FDA approval of our non-invasive blood glucose monitoring device as soon as possible. To guide us in that undertaking we previously announced the hiring of a Chief Medical Officer and formed a medical and regulatory advisory board to guide us through the FDA process. Additionally, we have retained third party quality assurance and documentation consultants to ensure that the rigorous requirements of the FDA are met. We are unable, however, to estimate the time necessary for FDA approval or the likelihood of success in that endeavor.
While the first focus of our Bio-RFID platform is non-invasive glucose monitoring, it is important to note that our KnowU and the UBand devices have the capacity to monitor and identify other analytes in the human body with a relatively simple software modification. Each additional analyte the Company identifies over time will require its own subsequent FDA approval, the success of which we are unable to estimate at this time.
Our ChromaID patented technology utilizes light at the photon (elementary particle of light) level through a series of emitters and detectors to generate a unique signature or “fingerprint” from a scan of almost any solid, liquid or gaseous material. This signature of reflected or transmitted light is digitized, creating a unique ChromaID signature. Each ChromaID signature is comprised of hundreds to thousands of specific data points.
The ChromaID technology looks beyond visible light frequencies to areas of near infra-red and ultraviolet light and beyond that are outside the humanly visible light spectrum. The data obtained allows us to create a very specific and unique ChromaID signature of the substance for a myriad of authentication, verification, and identification applications.
Bio-RFID, ChromaID and AI Deep Learning: Foundational Platform Technologies
Our technologies provide a unique platform upon which a myriad of applications can be developed. As platform technologies, they are analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. Bio-RFID and ChromaID technologies are “enabling” technologies that bring the science of electromagnetic energy to low-cost, real-world commercialization opportunities across multiple industries. The technologies are foundational and, as such, the basis upon which our company believes significant businesses can be built. While our company is pursuing our core focus on commercializing our glucose monitor, we believe non-core clinical and non-clinical applications represent a multitude of opportunities for strategic collaboration and joint development agreements with leading companies in their respective industries.
As with other foundational technologies, a single application may reach across multiple industries. The Bio-RFID technology can non-invasively identify the presence and quantity of blood glucose in the human body. By extension, there may be other analytes or molecular structures this same technology can identify in the human body which, over time, we intend to focus on. They may include the monitoring of drug usage or the presence of illicit drugs. They may also involve identifying hormones and various biomarkers of disease or pre-conditions of disease.
Similarly, the ChromaID technology can, for example, effectively differentiate and identify different brands of clear vodkas that appear identical to the human eye. By extension, this same technology could identify pure water from water with contaminants present. It could provide real time detection of liquid medicines such as morphine that have been adulterated or compromised. It could detect if jet fuel has water contamination present. It could determine when it is time to change oil in a deep fat fryer. These are but a few of the potential applications of the ChromaID technology based upon extensions of its ability to identify different liquids.
| 35 |
| Table of Contents |
The AI deep learning platform is an enabling technology which can identify patterns from data gathered from both the Bio-RFID and ChromaID platform technologies. The AI deep learning platform is critical to our company’s ability to identify accurately blood glucose levels and other analytes in the human body. Over time, utilizing our AI deep learning platform we plan to develop analytics which, when using data collected from our sensors, will provide useful information on health and wellness to end users, and potentially lead to what our company calls “Predictive Health.” In addition to identifying patterns, the inverse is also possible as our company’s AI deep learning platform can also create patterns in the form of 3D graphical images. That activity has found its first form in the work of our company’s subsidiary, AI Mind, to generate beautiful 3D graphical images which were sold as NFTs providing revenue in the first quarter of fiscal year 2022. Our company believes there will be future revenue generation from the sale of NFTs and from other applications of our AI deep learning platform.
The cornerstone of our foundational platform technology is our intellectual property portfolio. We have pursued an active intellectual property strategy which includes focus on patents where appropriate and a diligent protection of trade secrets. Our company has been granted 26 patents and 13 design patents. We currently have a number of patents pending and continue, on a regular basis, the filing of new patents. We possess all right, title and interest to the issued patents.
Product Strategy
We are currently undertaking internal development work on potential products for the commercial marketplace. We have announced the development of our non-invasive glucose monitor and our desire to obtain FDA clearance for the marketing of this product. We have also announced the engagement of a manufacturing partner we will work with to bring this product to market. We will make further announcements regarding this product as development, testing, manufacturing, and regulatory approval work progresses.
Currently we are focusing our efforts on productizing our Bio-RFID technology as we move it out of our research laboratory, through appropriate and required clinical trials and into the marketplace.
Our subsidiary corporation, Particle, Inc. is seeking a strategic distribution partner or partners to move its virus deactivating light bulb into the global marketplace. Our AI Mind subsidiary is looking at additional ways to monetize our AI deep learning platform beyond the NFT market for its graphical images and expects to test several product ideas over the next fiscal year.
Sales and Marketing
While we continues with our internal development efforts and the move toward FDA filing and expected (but not guaranteed) clearance of our first product, a non-invasive blood glucose monitoring device, we will explore the several potential avenues for moving our first product and potential follow on products into the marketplace. The avenues being explored include direct to consumer, initial launch partners, broad distribution partners, licensing partners and private label approaches to the market among others. We have begun to build our internal sales and marketing team in preparation for detailed strategic thinking about the optimal approach to the marketplace.
Competition
We group the competition into three large categories. Those are (i) large global technology companies who may enter the blood glucose monitoring and other diagnostic markets, (ii) legacy providers of blood glucose monitoring technology, and (iii) new entrants working to achieve a non-invasive solution or more acceptable blood glucose monitoring solution which may or may not be similar to our technology. With regard to companies in each category, we perform due diligence from all publicly available sources of information on their relevant technologies and their product plans. This information informs and refines our activities and underscores our sense of urgency as we work to bring our own technology to the marketplace. The addressable market is very large and there is room for a multitude of providers of blood glucose monitoring services. Of note, few, if any, of the competitors in the blood glucose monitoring space possess a platform technology competitive with our Bio-RFID technology and our ability to identify a multitude of analytes in the human body.
Our Competitive Advantage Bio-RFID’s ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so concurrently, and in real time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring.
|
| · | Non-Invasive: Our Bio-RFID technology is non-invasive, using radio waves to identify and measure what is going on inside the body. |
|
|
|
|
|
| · | Form Factor Agnostic: Our Bio-RFID technology platform that can be integrated into a variety of wearable, mobile or bench-top form factors. |
|
|
|
|
|
| · | Pain-free: No needles nor invasive transmitters in your body, making Bio-RFID sensors convenient and pain-free. |
|
|
|
|
|
| · | No Consumables: Expensive supplies, such as test strips and lancets, are not required to operate Bio-RFID devices. |
Our Growth Strategy
The key elements of our strategy to grow our business include:
|
| · | Initially, entering the diabetes continuous glucose monitoring, or CGM, market with our non-invasive CGM product. |
|
|
|
|
|
| · | Following our entry into the CGM market, entering other clinical monitoring markets for continuous, non-invasive hormone, medication metabolite, endocrinology components and biomolecular monitoring. |
|
|
|
|
|
| · | Applying our Bio-RFID platform technology to lifestyle analysis, clinical trials and chronic illnesses. |
We believe that potential use cases include real time wearable medication monitoring and detection of, for example, ovulation and hormone deficiency.
Research and Development
Our current research and development efforts are primarily focused on improving our Bio-RFID technology, extending its capacity, and developing new and unique applications for this technology and the AI deep learning platform that drives its analytics. As part of this effort, we conduct on-going laboratory testing to ensure that application methods are compatible with the end-user and regulatory requirements, and that they can be implemented in a cost-effective manner. We are also actively involved in identifying new applications. Our current internal team along with outside consultants have considerable experience working with the application of our technologies and their application. We engage third party experts as required to supplement our internal team. We believe that continued development of new and enhanced technologies is essential to our future success. We incurred expenses of $3,406,996, $3,969,972 and $2,033,726 for the nine months ended June 30, 2022 and the years ended September 30, 2021 and 2020, respectively, on development activities.
Our wholly owned subsidiary, Particle, Inc. is focused on the development and commercialization of our extensive intellectual property relating to electromagnetic energy outside of the medical diagnostic arena which remains our singular focus. Since its incorporation, Particle has engaged in research and development activities on threaded light bulbs that have a warm white light and can inactivate germs, including bacteria and viruses. Particle is now looking for partners to commercialize this product.
Our wholly owned subsidiary, AI Mind is focused on monetizing our AI deep learning platform. Since its incorporation AI Mind has focused on creating patterns from our company data, which were sold as NFTs. Our company will continue to look for opportunities for new applications on our AI deep learning platform, to generate revenues to support the continued development of our non-invasive diagnostic technology.
Our Patents and Intellectual Property
We believe that our 27 patents and 13 design patents, patent applications, registered trademarks, and our trade secrets, copyrights and other intellectual property rights are important assets. Our issued patents will expire at various times between 2027 and 2041. Pending patents, if and when issued, may have expiration dates that extend further in time. The duration of our trademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
The issued patents cover the fundamental aspects of the Know Labs ChromaID and Bio-RFID technology and a number of unique applications. We have filed patents on the fundamental aspects of our Bio-RFID technology and growing number of unique applications. We will continue to expand our company’s patent portfolio.
Additionally, significant aspects of our technology are maintained as trade secrets which may not be disclosed through the patent filing process. We are diligent in maintaining and securing our trade secrets.
Related Patent Assets
Inherent in a platform technology is the ability to develop or license technology in diverse fields of use apart from our company’s core focus. We focus on human health and wellness with a first focus on the non-invasive monitoring of blood glucose. We will pursue the identification of a multitude of analytes in the human body important to diagnostics over time. We will also identify, over time, opportunities for our intellectual property to be deployed in areas outside human health and wellness. Examples are Particle and AI Mind.
| 36 |
| Table of Contents |
Our wholly owned subsidiary, Particle, is engaged in research and development on non-core Company intellectual property. The first research activity, undertaken by Particle has been related to standard threaded light bulbs that emit a warm white light that can inactivate germs, including bacteria and viruses. On May 21, 2020, we entered into an intercompany patent license agreement with Particle pursuant to which Particle received an exclusive non-transferrable license to use certain of our patents and trademarks. In exchange for this license, we will receive: (i) a one-time fee of $250,000 upon a successful financing of Particle, and (ii) a quarterly royalty payment equal to the greater of 5% of the gross sales, net of returns, from Particle and $5,000. As of June 30, 2022, the operations of Particle have not generated any sales. The first product, the Particle bulb, can be used in households, businesses, and other facilities to inactivate bacteria and viruses. Through internal preliminary testing, Particle personnel have confirmed the Particle bulb’s efficacy in inactivating common germs such as E. coli and Staphylococcus. Final study results from Texas Biomedical Research Institute indicate that the Particle bulb has the ability to inactivate SARS-CoV-2, the virus that causes COVID-19 and, most recently, the Alpha and Delta variants of the COVID-19 virus. The Particle team is working on certification, labeling, product manufacturing and related go-to-market requirements as well as business development activities related to interest from potential strategic and channel partners in both consumer and business applications in the global marketplace.
We expect although we cannot guarantee that we will create other such subsidiaries over time. Additionally, we may license our intellectual property to third parties so that they may pursue activities that are not a part of our company’s core focus.
Our Facilities
Corporate Offices
On April 13, 2017, we leased our executive office located at 500 Union Street, Suite 810, Seattle, Washington, USA, 98101. The Company leases 943 square feet and the current net monthly payment is $3,334. The monthly payment increases approximately 3% each year and the lease expires on May 31, 2022. On October 31, 2021, we extended the lease from June 1, 2022 to May 31, 2023 at $2,986 per month.
Lab Facilities and Executive Offices
On February 1, 2019, we leased its lab facilities and executive offices located at 915 E Pine Street, Suite 212, Seattle, WA 98122. We lease 2,642 square feet and the net monthly payment at September 30, 2021 is $8,697. The monthly payment increases approximately 3% annually each year on July 1. The lease expires on June 30, 2024. On October 11, 2021, the Company entered into First Amendment of Lease and added 1,030 square feet for year for $5,000 per month. The space will be utilized for clinical trials.
Employees
As of June 30, 2022, we had 15 full-time employees. Our senior management and 10 other personnel are located in our Seattle, Washington offices. We periodically utilize consulting firms and individual contractors to supplement our workforce.
Government Regulation
United States
Our medical diagnostic products and operations, initially the KnowU and UBand glucose monitoring products, are subject to extensive and rigorous regulation by the U.S. Food and Drug Administration, or FDA, under the Federal Food, Drug, and Cosmetic Act, or FFDCA, and its implementing regulations, guidance documentation, and standards. Our KnowU and UBand products will be regulated by the FDA as medical devices. The FDA regulates the design, development, research, testing, manufacturing, safety, labeling, storage, recordkeeping, promotion, distribution, sale and advertising of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. The FDA also regulates the export of medical devices manufactured in the United States to international markets. Any violations of these laws and regulations could result in a material adverse effect on our business, financial condition and results of operations. In addition, if there is a change in law, regulation or judicial interpretation, we may be required to change our business practices, which could have a material adverse effect on our business, financial condition and results of operations.
Under the FFDCA, medical devices are classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device and the extent of control needed to ensure safety and effectiveness.
| 37 |
| Table of Contents |
Class I devices are those for which safety and effectiveness can be assured by adherence to FDA’s “general controls” for medical devices, which include compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. Some Class I devices also require premarket clearance by the FDA through the 510(k) premarket notification process described below.
Class II devices are subject to FDA’s general controls, and any other “special controls” deemed necessary by FDA to ensure the safety and effectiveness of the device, such as performance standards, product-specific guidance documents, special labeling requirements, patient registries or post-market surveillance. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification procedure, though certain Class II devices are exempt from this premarket review process. When a 510(k) is required, the manufacturer must submit to the FDA a premarket notification submission demonstrating that the device is “substantially equivalent” to a legally marketed device, which in some cases may require submission of clinical data. Unless a specific exemption applies, 510(k) premarket notification submissions are subject to user fees. If the FDA determines that the device, or its intended use, is not substantially equivalent to a legally marketed device, the FDA will place the device, or the particular use of the device, into Class III, and the device sponsor must then fulfill much more rigorous premarketing requirements.
Class III devices, consisting of devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device. The safety and effectiveness of Class III devices cannot be assured solely by general or special controls. Submission and FDA approval of a premarket approval, or PMA, application is required before marketing of a Class III device can proceed. As with 510(k) submissions, unless subject to an exemption, PMA submissions are subject to user fees. The PMA process is much more demanding than the 510(k) premarket notification process. A PMA application, which is intended to demonstrate that the device is safe and effective, must be supported by extensive data, typically including data from preclinical studies and human clinical trials.
510(k) Clearance
To obtain 510(k) clearance for a medical device, an applicant must submit to the FDA a premarket notification submission demonstrating that the proposed device is “substantially equivalent” to a legally marketed device, known as a “predicate device.” A legally marketed predicate device may include a device that was legally marketed prior to May 28, 1976 for which a PMA is not required (known as a ”pre-amendments device” based on the date of enactment of the Medical Device Amendments of 1976), a device that has been reclassified from Class III to Class II or Class I, or a device that was found substantially equivalent through the 510(k) process. A device is substantially equivalent if, with respect to the predicate device, it has the same intended use and has either (i) the same technological characteristics, or (ii) different technological characteristics, but the information provided in the 510(k) submission demonstrates that the device does not raise new questions of safety and effectiveness and is at least as safe and effective as the predicate device. A showing of substantial equivalence sometimes, but not always, requires clinical data.
Before the FDA will accept a 510(k) submission for substantive review, the FDA will first assess whether the submission satisfies a minimum threshold of acceptability. If the FDA determines that the 510(k) submission is incomplete, the FDA will issue a “Refuse to Accept” letter which generally outlines the information the FDA believes is necessary to permit a substantive review and to reach a determination regarding substantial equivalence. An applicant must submit the requested information before the FDA will proceed with additional review of the submission. Once the 510(k) submission is accepted for review, by regulation, the FDA has 90 days to review and issue a determination. As a practical matter, clearance often takes longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence.
If the FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant 510(k) clearance to commercially market the device. If the FDA determines that the device is “not substantially equivalent” to a previously cleared device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process, which is a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device.
After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, PMA approval. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance. Many minor modifications today are accomplished by a “letter to file” in which the manufacture documents the rationale for the change and why a new 510(k) is not required. However, the FDA may review such letters to file to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
| 38 |
| Table of Contents |
PMA Approval
A PMA must be submitted to the FDA for any device that is classified in Class III or otherwise cannot be cleared through the 510(k) process (although the FDA has discretion to continue to allow certain pre-amendment Class III devices to use the 510(k) process). PMA applications must be supported by, among other things, valid scientific evidence demonstrating the safety and effectiveness of the device, which typically requires extensive data, including technical, preclinical, clinical and manufacturing data. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities, and controls used for manufacturing, and proposed labeling. Following receipt of a PMA application, once the FDA determines that the application is sufficiently complete to permit a substantive review, the FDA will formally accept the application for review. The FDA, by statute and by regulation, has 180-days to review an “accepted” PMA application, although the review of an application more often occurs over a significantly longer period of time, and can take up to several years. During the review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the QSR.
If the FDA evaluations of both the PMA application and the manufacturing facilities are favorable, the FDA will either issue an approval letter or an approvable letter, which usually contains a number of conditions that must be met in order to secure final approval of the PMA. If the FDA’s evaluation of the PMA or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. A not approvable letter will outline the deficiencies in the application and, where practical, will identify what is necessary to make the PMA approvable. The FDA may also determine that additional clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted. Once granted, PMA approval may be withdrawn by the FDA if compliance with post-approval requirements, conditions of approval or other regulatory standards is not maintained or problems are identified following initial marketing.
In approving a PMA the FDA may also require some form of post-market surveillance when necessary to protect the public health or to provide additional safety and effectiveness data for the device. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and makes periodic reports to the FDA on the clinical status of those patients.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of a PMA-approved device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel.
De Novo Classification
Medical device types that the FDA has not previously classified as Class I, II or III are automatically classified into Class III regardless of the level of risk they pose. The Food and Drug Administration Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, called the “Request for Evaluation of Automatic Class III Designation,” or the de novo classification procedure. This procedure allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. Under FDASIA, the FDA is required to classify the device within 120 days following receipt of the de novo application. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, the FDA may reject the reclassification petition if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed.
It is our current belief that our initial product is appropriate for a de novo classification request.
| 39 |
| Table of Contents |
Breakthrough Devices Program
The Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency’s mission to protect and promote public health.
The Breakthrough Devices Program replaces the Expedited Access Pathway and Priority Review for medical devices. The FDA considers devices granted designation under the Expedited Access Pathway to be part of the Breakthrough Devices Program.
The Company may pursue the Breakthrough Devices Program.
Clinical Studies
When FDA clearance or approval of a Class I, Class II or Class III device requires human clinical trials, and if the device presents a “significant risk” to human health, the device sponsor is required to file an IDE application with the FDA and obtain IDE approval prior to commencing the human clinical trial. If the device is considered a ”non-significant risk,” IDE submission to FDA is not required. Instead, only approval from the Institutional Review Board, or IRB, overseeing the investigation at each clinical trial site is required. Human clinical studies are generally required in connection with approval of Class III devices and may be required for Class I and II devices. The FDA or the IRB at each institution at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product in the United States.
Continuing Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
|
| · | Product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
|
|
|
|
|
| · | QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; |
|
|
|
|
|
| · | labeling regulations and FDA prohibitions against the promotion of products for uncleared or unapproved ”off-label” uses; |
|
|
|
|
|
| · | clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; |
|
|
|
|
|
| · | approval of product modifications that affect the safety or effectiveness of one of our approved devices; |
|
|
|
|
|
| · | medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
|
|
|
|
|
| · | post-approval restrictions or conditions, including post-approval study commitments; |
|
|
|
|
|
| · | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
|
|
|
|
|
| · | the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
|
|
|
|
|
| · | regulations pertaining to voluntary recalls; and |
|
|
|
|
|
| · | notices of corrections or removals. |
| 40 |
| Table of Contents |
Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. If the FDA determines that our promotional materials or training constitutes promotion of an unapproved or uncleared use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved or uncleared use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged, and adoption of the products would be impaired.
Furthermore, our products could be subject to voluntary recall if we or the FDA determine, for any reason, that our products pose a risk of injury or are otherwise defective. Moreover, the FDA can order a mandatory recall if there is a reasonable probability that our product would cause serious adverse health consequences or death.
The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of some of our subcontractors. Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other regulatory authorities, which may result in sanctions including, but not limited to:
|
| · | Untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
|
|
|
|
| · | unanticipated expenditures to address or defend such actions; |
|
|
|
|
|
| · | customer notifications for repair, replacement, refunds; |
|
|
|
|
|
| · | recall, detention or seizure of our products; |
|
|
|
|
|
| · | operating restrictions or partial suspension or total shutdown of production; |
|
|
|
|
|
| · | refusing or delaying our requests for 510(k) clearance or PMA approval of new products or modified products; |
|
|
|
|
|
| · | withdrawing 510(k) clearances or PMA approvals that have already been granted; |
|
|
|
|
|
| · | refusal to grant export approval for our products; or |
|
|
|
|
|
| · | criminal prosecution. |
International
Our international sales are subject to regulatory requirements in the countries in which our products are sold. The regulatory review process varies from country to country and may in some cases require the submission of clinical data.
| 41 |
| Table of Contents |
Directors and Executive Officers
Set forth below is information regarding our directors and executive officers as of the date of this prospectus.
| Name |
| Age |
| Position |
| Ronald P. Erickson |
| 78 |
| Chairman |
| Phillip A. Bosua |
| 48 |
| Chief Executive Officer and Director |
| Peter Conley |
| 67 |
| Chief Financial Officer and SVP Intellectual Property |
| William A. Owens |
| 82 |
| Director |
| Jon Pepper |
| 71 |
| Director |
| Ichiro Takesako |
| 63 |
| Director |
Ronald P. Erickson has been a director and officer of Know Labs since April 2003. He was appointed as our CEO and President in November 2009 and as Chairman of the Board in February 2015. He served as our CEO until April 2018. Previously, Mr. Erickson was our President and Chief Executive Officer from September 2003 through August 2004 and was Chairman of the Board from August 2004 until May 2011. Mr. Erickson stepped down as Chief Executive Officer on April 10, 2018. A senior executive with more than 30 years of experience in the high technology, telecommunications, micro-computer, and digital media industries, Mr. Erickson was the founder of Know Labs. He is formerly Chairman, CEO and Co-Founder of Blue Frog Media, a mobile media and entertainment company; Chairman and CEO of eCharge Corporation, an Internet-based transaction procession company, Chairman, CEO and Co-founder of GlobalTel Resources, a provider of telecommunications services; Chairman, Interim President and CEO of Egghead Software, Inc. a software reseller where he was an original investor; Chairman and CEO of NBI, Inc.; and Co-founder of MicroRim, Inc. the database software developer. Earlier, Mr. Erickson practiced law in Seattle and worked in public policy in Washington, DC and New York, NY. Additionally, Mr. Erickson has been an angel investor and board member of a number of public and private technology companies. In addition to his business activities, Mr. Erickson is Chairman of the Board of Trustees of Central Washington University where he received his BA degree. He also holds a MA from the University of Wyoming and a JD from the University of California, Davis. He is licensed to practice law in the State of Washington. Mr. Erickson is our founder and was appointed as a director because of his extensive experience in developing technology companies.
Phillip A. Bosua was appointed a director and Chief Executive Officer of our company on April 10, 2018. Previously, Mr. Bosua served as our Chief Product Officer since August 2017 and we entered into a Consulting Agreement on July 7, 2017. From September 2012 to February 2015, he was the founder and Chief Executive Officer of LIFX Inc. (where he developed and marketed an innovative “smart” light bulb) and from August 2015 until February 2016 was Vice President Consumer Products at Soraa (which markets specialty LED light bulbs). From February 2016 to July 2017, Mr. Bosua was the founder and CEO of RAAI, Inc. (where he continued the development of his smart lighting technology). From May 2008 to February 2013, he was the Founder and CEO of LimeMouse Apps, a leading developer of applications for the Apple App Store. Mr. Bosua was appointed as a director because of his extensive experience in developing technology companies.
Peter Conley has served as our Chief Financial Officer and SVP Intellectual Property (IP) since May 20, 2022. In addition, Mr. Conley currently serves as Senior Managing Director and Head of Intellectual Property Banking at Boustead Securities, LLC since October 2014, where he provides equity financing and M&A advisory services to small-cap public companies. Prior to that, from 2012 to 2016, Mr. Conley was a cofounder and Chief Operating Officer of ipCreate, a global IP development and innovation services company serving large multinational companies. He also served as managing director of ipCapital Venture Group, where he provided IP strategy and venture advisory services. During his career spanning more than 35 years, Mr. Conley has held leadership roles at MDB Capital Group, The Analytiq Group / RDEX Research, Roth Capital Partners, and Lehman Brothers. He was on the founding team and Head of Equity Capital Markets at E*Offering, the investment bank of E*Trade. Mr. Conley attended the University of Hawaii at Manoa and the University of London, Center for Financial & Management Studies, SOAS.
| 42 |
| Table of Contents |
William A. Owens has served as an independent director since May 24, 2018. Admiral William A. Owens is currently the co- founder and executive chairman of Red Bison Advisory Group, a company which identifies opportunities with proven enterprises in China, the Middle East, and the United States and creates dynamic partnerships focusing on natural resources (oil, gas and fertilizer plants), real estate, and information and communication technology. Most recently, he was the chairman of the board of CenturyLink Telecom, the third largest telecommunications company in the United States and was on the advisory board of SAP USA. Owens serves on the board of directors at Wipro Technologies and Know Labs Inc. Owens is on the advisory board of the following private companies: Carillon Technologies, Platform Science, Prism, Sarcos, Sierra Nevada Corporation and Vodi. Owens is on the board of trustees at EastWest Institute, Seattle University, and an advisor to the Post COVID-19 Debt Initiative (PCDI). He is also a member of the Council of Foreign Relations. From 2007 to 2015, Owens was the Chairman and Senior Partner of AEA Investors Asia, a private equity firm located in Hong Kong, and Vice Chairman of the NYSE for Asia. Owens also served as the Chairman of Eastern Airlines. He has served on over 20 public boards including Daimler, British American Tobacco, Telstra, Nortel Networks, and Polycom. Owens was the CEO/Chairman of Teledesic LLC, a Bill Gates/Craig McCaw company bringing worldwide broadband through an extensive satellite network and prior, was the President, COO/Vice Chairman of Science Applications International Corporation (SAIC). Owens has also served on the boards of the non-for-profit organizations; Fred Hutchinson Cancer Research Center, Carnegie Corporation of New York, Brookings Institution, and RAND Corporation. Owens is a four-star US Navy veteran. He was Vice Chairman of the Joint Chiefs of Staff, the second-ranking United States military officer with responsibility for reorganizing and restructuring the armed forces in the post- Cold War era. He is widely recognized for bringing commercial high-grade technology into the Department of Defense for military applications. Owens was the architect of the Revolution in Military Affairs (RMA), an advanced systems technology approach to military operations, the most significant change in the system of requirements, budgets and technology for the four-armed forces since World War II. Owens, served as Commander of the U.S. Sixth Fleet from 1990 to 1992, which included Operation Desert Storm. Owens also served as the deputy chief of Naval Operations for Resources. Owens was senior military assistant to two Secretaries of Defense (Cheney and Carlucci) and served in the Office of Program Appraisal for the Secretary of the Navy. He began his military career as a nuclear submariner. He served on four strategic nuclear-powered submarines and three nuclear attack submarines, including tours as Commanding Officer aboard the USS Sam Houston, Michigan, and USS City of Corpus Christi. Owens spent a total of 2000 days submerged aboard submarines, including duty in Vietnam. Owens is a 1962 honor graduate of the United States Naval Academy with a bachelor’s degree in mathematics, bachelor’s and master’s degrees in politics, philosophy and economics from Oxford University, and a master’s degree in management from George Washington University. He has written more than fifty articles on national security and authored the book “High Seas.” His book, “Lifting the Fog of War,” was published in April 2000 with a revision published in Mandarin in 2009. Owens has received numerous recognitions and awards: the “Légion d’Honneur” by France, and the highest awards given to foreigners by the countries of Indonesia and Sweden. He was named as one of The 50 Most Powerful People in Networking by Network World, one of the one hundred Best Board Members in the United States for 2011 and again in 2016 awarded by NACD, awarded the David Sarnoff Award for Technology Innovation and the Intrepid Salute Award in recognition of his business achievements and support of important philanthropic activities. Owens is active in philanthropy to foster Chinese – American relations including dialogues between the most senior retired officers in the United States and Chinese militaries and similar dialogues between very senior economists. He is one of North Dakota’s Roughriders recipients, the award given annually to some of the most prominent North Dakotans.
Jon Pepper has served as an independent director since April 2006. Mr. Pepper founded Pepcom in 1980, a company that become the industry leader at producing press-only technology showcase events around the country and internationally. He sold his stake in the corporation and retired as a partner at the end of 2018. Prior to that, Mr. Pepper started the DigitalFocus newsletter, a ground-breaking newsletter on digital imaging that was distributed to leading influencers worldwide. Mr. Pepper has been closely involved with the high technology revolution since the beginning of the personal computer era. He was formerly a well-regarded journalist and columnist; his work on technology subjects appeared in The New York Times, Fortune, PC Magazine, Men’s Journal, Working Woman, PC Week, Popular Science and many other well-known publications. Pepper was educated at Union College in Schenectady, New York and the Royal Academy of Fine Arts in Copenhagen. He continues to be active in non-profit work and boards, and in 2017 founded Mulberry Tree Films, a non-profit that supports independent high-quality documentary films. The company funded and produced the acclaimed documentary, “The Gates of Shinto” and is looking into additional projects. Mr. Pepper was appointed as a director because of his marketing skills with technology companies.
Ichiro Takesako has served as a director since December 28, 2012. Mr. Takesako has held executive positions with Sumitomo Precision Products Co., Ltd, or Sumitomo, and its affiliates since 1983. Mr. Takesako graduated from Waseda University, Tokyo, Japan where he majored in Social Science and graduated with a Degree of Bachelor of Social Science.
| 43 |
| Table of Contents |
In the past few years, Mr. Takesako has held the following executive position in Sumitomo and its affiliates:
| June 2008: | appointed as General Manager of Sales and Marketing Department of Micro Technology Division |
|
|
|
| April 2009: | appointed as General Manager of Overseas Business Department of Micro Technology Division, in charge of M&A activity of certain business segment and assets of Aviza Technology, Inc. |
|
|
|
| July 2010: | appointed as Executive Director of SPP Process Technology Systems, 100% owned subsidiary of Sumitomo Precision Products then, stationed in Newport, Wales |
|
|
|
| August 2011: | appointed as General Manager, Corporate Strategic Planning Group |
|
|
|
| January 2013: | appointed as Chief Executive Officer of M2M Technologies, Inc., a company invested by Sumitomo Precision products |
|
|
|
| April 2013: | appointed as General Manager of Business Development Department, in parallel of CEO of M2M Technologies, Inc. |
|
|
|
| April 2014: | relieved from General Manager of Business Development Department and is responsible for M2M Technologies Inc. as its CEO |
|
|
|
| March 2017: | Established At Signal, Inc., took over the entire business operation from M2M Technologies, Inc. |
|
|
|
| April 2017: | appointed as Chief Executive Officer of At Signal Inc., a company invested by himself. |
| 44 |
| Table of Contents |
Mr. Takesako was appointed as a director based on his previous position with Sumitomo and Sumitomo’s previous significant partnership with our company.
Our directors currently have terms which will end at our next annual meeting of the stockholders or until their successors are elected and qualify, subject to their prior death, resignation or removal. Officers serve at the discretion of the board of directors.
Family Relationships
There are no family relationships among any of our officers or directors.
Involvement in Certain Legal Proceedings
To the best of our knowledge, except as described below, none of our directors or executive officers has, during the past ten years:
|
| · | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
|
|
|
|
|
| · | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
|
|
|
|
|
| · | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
|
|
|
|
|
| · | been found by a court of competent jurisdiction in a civil action or by the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
|
|
|
|
|
| · | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
|
|
|
|
|
| · | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Corporate Governance
Our Board’s Role in Risk Oversight
Our board of directors oversees that the assets of our company are properly safeguarded, that the appropriate financial and other controls are maintained, and that our business is conducted wisely and in compliance with applicable laws and regulations and proper governance. Included in these responsibilities is the board’s oversight of the various risks facing our company. In this regard, our board seeks to understand and oversee critical business risks. Our board does not view risk in isolation. Risks are considered in virtually every business decision and as part of our business strategy. Our board recognizes that it is neither possible nor prudent to eliminate all risk. Indeed, purposeful and appropriate risk-taking is essential for our company to be competitive on a global basis and to achieve our objectives.
While the board oversees risk management, company management is charged with managing risk. Management communicates routinely with the board and individual directors on the significant risks identified and how they are being managed. Directors are free to, and indeed often do, communicate directly with senior management.
Our board administers its risk oversight function as a whole by making risk oversight a matter of collective consideration. Much of this work has been delegated to committees, which will meet regularly and report back to the full board. The audit committee oversees risks related to our financial statements, the financial reporting process, accounting and legal matters, the compensation committee evaluates the risks and rewards associated with our compensation philosophy and programs, and the nominating and corporate governance committee evaluates risk associated with management decisions and strategic direction.
Independent Directors
For purposes of determining independence, we have adopted the definition of independence as contained in NYSE American’s rules. Pursuant to the definition, we have determined that as of the date hereof, that Messrs. Owens, Pepper and Takesako qualify as independent. Our board of directors currently consists of five (5) directors, three (3) of whom are independent within the meaning of NYSE American rules.
Committees of the Board of Directors
Our board of directors has established three standing committees: (1) an Audit Committee, (2) a Nominating and Corporate Governance Committee, and (3) a Compensation Committee. These committees were formed in July 2010. Each of the committees operates under a written charter adopted by the board of directors. These charters are available on our website at www.knowlabs.co. In addition, our board of directors may, from time to time, designate one or more additional committees, which shall have the duties and powers granted to it by our board of directors.
Audit Committee
William A. Owens Jon Pepper and Ichiro Takesako, each of whom satisfies the “independence” requirements of Rule 10A-3 under the Exchange Act and NYSE American’s rules, serve on our audit committee, with Mr. Pepper serving as the chairman. Our board has determined that Mr. Owens qualifies as an “audit committee financial expert.” The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company.
The audit committee is responsible for, among other things: (i) retaining and overseeing our independent accountants; (ii) assisting the board in its oversight of the integrity of our financial statements, the qualifications, independence and performance of our independent auditors and our compliance with legal and regulatory requirements; (iii) reviewing and approving the plan and scope of the internal and external audit; (iv) pre-approving any audit and non-audit services provided by our independent auditors; (v) approving the fees to be paid to our independent auditors; (vi) reviewing with our chief executive officer and principal financial officer and independent auditors the adequacy and effectiveness of our internal controls; (vii) reviewing hedging transactions; and (viii) reviewing and assessing annually the audit committee’s performance and the adequacy of its charter.
Compensation Committee
William A. Owens, Jon Pepper and Ichiro Takesako, each of whom satisfies the “independence” requirements of Rule 10C-1 under the Exchange Act and NYSE American’s rules, serve on our compensation committee, with Mr. Owens serving as the chairman. The members of the compensation committee are also “non-employee directors” within the meaning of Section 16 of the Exchange Act. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers.
| 45 |
| Table of Contents |
The compensation committee is responsible for, among other things: (i) reviewing and approving the remuneration of our executive officers; (ii) making recommendations to the board regarding the compensation of our independent directors; (iii) making recommendations to the board regarding equity-based and incentive compensation plans, policies and programs; and (iv) reviewing and assessing annually the compensation committee’s performance and the adequacy of its charter.
Nominating and Corporate Governance Committee
William A. Owens, Jon Pepper and Ichiro Takesako, each of whom satisfies the “independence” requirements of NYSE American’s rules serve on our nominating and corporate governance committee, with Mr. Pepper serving as the chairman. The nominating and corporate governance committee assists the board of directors in selecting individuals qualified to become our directors and in determining the composition of the board and its committees.
The nominating and corporate governance committee will be responsible for, among other things: (i) identifying and evaluating individuals qualified to become members of the board by reviewing nominees for election to the board submitted by stockholders and recommending to the board director nominees for each annual meeting of stockholders and for election to fill any vacancies on the board; (ii) advising the board with respect to board organization, desired qualifications of board members, the membership, function, operation, structure and composition of committees (including any committee authority to delegate to subcommittees), and self-evaluation and policies; (iii) advising on matters relating to corporate governance and monitoring developments in the law and practice of corporate governance; (iv) overseeing compliance with the our code of ethics; and (v) approving any related party transactions.
The nominating and corporate governance committee’s methods for identifying candidates for election to our board of directors (other than those proposed by our stockholders, as discussed below) will include the solicitation of ideas for possible candidates from a number of sources - members of our board of directors, our executives, individuals personally known to the members of our board of directors, and other research. The nominating and corporate governance committee may also, from time-to-time, retain one or more third-party search firms to identify suitable candidates.
In making director recommendations, the nominating and corporate governance committee may consider some or all of the following factors: (i) the candidate’s judgment, skill, experience with other organizations of comparable purpose, complexity and size, and subject to similar legal restrictions and oversight; (ii) the interplay of the candidate’s experience with the experience of other board members; (iii) the extent to which the candidate would be a desirable addition to the board and any committee thereof; (iv) whether or not the person has any relationships that might impair his or her independence; and (v) the candidate’s ability to contribute to the effective management of our company, taking into account the needs of our company and such factors as the individual’s experience, perspective, skills and knowledge of the industry in which we operate.
A stockholder may nominate one or more persons for election as a director at an annual meeting of stockholders if the stockholders comply with the notice and information provisions contained in our bylaws. Such notice must be in writing to our Company not later than the close of business on the ninetieth (90th) day nor earlier than the close of business on the one-hundred-twentieth (120th) day prior to the first anniversary of the preceding year’s annual meeting; provided, however, that in the event that the date of the annual meeting is advanced more than thirty (30) days prior to or delayed by more than thirty (30) days after the anniversary of the preceding year’s annual meeting, notice by the stockholder to be timely must be so delivered not earlier than the close of business on the one hundred twentieth (120th) day prior to such annual meeting and not later than the close of business on the later of the ninetieth (90th) day prior to such annual meeting or the tenth (10th) day following the day on which public announcement of the date of such meeting is first made or as otherwise required by the Exchange Act. In addition, stockholders furnishing such notice must be a holder of record on both (i) the date of delivering such notice and (ii) the record date for the determination of stockholders entitled to vote at such meeting.
Code of Ethics
We have adopted a code of ethics that applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer. Such code of ethics addresses, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, and reporting of violations of the code.
A copy of the code of ethics has been filed as an exhibit to the registration statement of which this prospectus is a part and is also available on our website as www.knowlabs.io. We are required to disclose any amendment to, or waiver from, a provision of our code of ethics applicable to our principal executive officer, principal financial officer, principal accounting officer, controller, or persons performing similar functions. We intend to use our website as a method of disseminating this disclosure as well as by SEC filings, as permitted or required by applicable SEC rules. Any such disclosure will be posted to our website within four (4) business days following the date of any such amendment to, or waiver from, a provision of our code of ethics.
| 46 |
| Table of Contents |
Summary Compensation Table - Years Ended September 30, 2021 and 2020
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named persons for services rendered in all capacities during the noted periods. No other executive officers received total annual salary and bonus compensation in excess of $100,000.
| Name and Principal Position |
| Year |
| Salary ($) |
|
| Bonus ($) |
|
| Stock Awards ($)(3) |
|
| Option Awards ($)(3) |
|
| Total ($) |
|
|||||
| Ronald P. Erickson, |
| 2021 |
|
| 366,042 |
|
|
| - |
|
|
| - |
|
|
| 1,811,691 |
|
|
| 2,177,733 |
|
| Chairman of the Board and Interim Chief Financial Officer (1) |
| 2020 |
|
| 243,333 |
|
|
| - |
|
|
| 190,000 |
|
|
| 394,000 |
|
|
| 827,333 |
|
| Phillip A. Bosua, |
| 2021 |
|
| 413,760 |
|
|
| 250,000 |
|
|
| - |
|
|
| - |
|
|
| 663,760 |
|
| Chief Executive Officer (2) |
| 2020 |
|
| 288,333 |
|
|
| - |
|
|
| 285,000 |
|
|
| 394,000 |
|
|
| 967,333 |
|
(1) During the years ended September 30, 2021 and 2020, our compensation committee and board of directors compensated Ronald P. Erickson, Chairman of the board of directors and, previously, Interim Chief Financial Officer, with an annual salary of $195,000 from October 1, 2019 to May 1, 2020. From May 1, 2020 to March 31, 2021, the annual compensation was $215,000. From April 1, 2021 to September 30, 2021, the annual compensation was $300,000. The compensation committee and the board of directors of our subsidiary, Particle, compensated Ronald P. Erickson with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. On December 15, 2020, we issued a fully vested warrant to Ronald P. Erickson exercisable for 2,000,000 shares of our common stock. The five-year warrant is exercisable for cash or non-cash at $1.53 per share and was valued using a Black-Scholes model at $1,811,691.The 100,000 shares of our restricted common stock issued on January 1, 2020 to Mr. Erickson were valued at the grant date market value of $1.90 per share. The stock grant was authorized at $0.17 per share. During the year end September 30, 2020, Mr. Erickson received a vested stock option grant from Particle for 500,000 shares of Particle common stock valued at $0.788 per share, or $394,000.
(2) During the years ended September 30, 2021 and 2020, our compensation committee and board of directors compensated Phillip A. Bosua, our Chief Executive Officer, with an annual salary of $240,000 from October 1, 2019 to May 1, 2020. From May 1, 2020 to March 31, 2021, Mr. Bosua’s annual compensation was $260,000. From April 1, 2021 to September 30, 2021, his annual compensation was $350,000. The compensation committee and the board of directors of Particle compensated Mr. Bosua with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. On March 18, 2021, we approved a $250,000 bonus for Mr. Bosua. The bonus was paid during April 2021.The 150,000 shares of restricted common stock issued on January 1, 2020 to Mr. Bosua were valued at the grant date market value of $1.90 per share. Mr. Bosua received a vested stock option grant from Particle for 500,000 shares of Particle common stock valued at $0.788 per share, or $394,000.
(3) These amounts reflect the grant date market value computed in accordance with FASB ASC Topic 718.
Employment Agreements
Phillip A. Bosua, Chief Executive Officer
On April 10, 2018, we entered into an employment agreement with Mr. Bosua reflecting his appointment as Chief Executive Officer. The employment agreement is for an initial term of 12 months (subject to earlier termination) and will be automatically extended for additional 12-month terms unless either party notifies the other party of its intention to terminate the Employment Agreement with at least ninety (90) days prior to the end of the Initial Term or renewal term. Mr. Bosua was paid a base salary of $225,000 per year, received 500,000 shares of common stock valued at $0.33 per share and may be entitled to bonuses and equity awards at the discretion of the Board or a committee of the Board. The employment agreement provides for severance pay equal to 12 months of base salary if Mr. Bosua is terminated without “cause” or voluntarily terminates his employment for “good reason.” During the years ended September 30, 2021 and 2020, our compensation committee and our board of directors compensated Mr. Bosua with an annual salary of $240,000 from October 1, 2019 to May 1, 2020. From May 1, 2020 to March 31, 2021, the annual compensation was $260,000. From April 1, 2021 to September 30, 2021, the annual compensation was $350,000. The compensation committee and the board of directors of Particle compensated Phillip A. Bosua with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. Mr. Bosua will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. If our company terminates Mr. Bosua’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Bosua terminates his employment at any time for “good reason” or due to a “disability,” Mr. Bosua will be entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months.
| 47 |
| Table of Contents |
Peter Conley, Chief Financial Officer and Senior Vice President, Intellectual Property
On May 13, 2022, we entered into an employment agreement with Mr. Conley reflecting his appointment as our Chief Financial Officer and Senior Vice President, Intellectual Property. The employment agreement is at will, meaning either we or Mr. Conley may terminate the employment relationship at any time, with or without cause, upon written notice to the other party. Under the terms of this agreement, Mr. Conley has an annualized base salary of $300,000, and the base salary will be paid periodically in accordance with our normal payroll practice. Mr. Conley may also be entitled to bonuses from time to time as determined by our board of directors or our Compensation Committee in their sole discretion. The employment agreement provides that Mr. Conley is eligible for equity awards under our 2021 Equity Incentive Plan or by agreement outside the plan. The employment agreement provides for severance pay equal to 12 months of then-in-effect base salary if Mr. Conley is terminated without “cause” or voluntarily terminates his employment for “good reason,” as defined in the employment agreement. Mr. Conley is eligible to participate in of all our employee benefit plans, policies and arrangements that are applicable to other executive officers, as such plans, policies and arrangements may exist or change from time to time at our discretion. We will reimburse Mr. Conley for reasonable travel, entertainment and other expenses he incurs in the furtherance of his duties under this agreement.
Ronald P. Erickson, Chairman of the Board
On April 10, 2018, we entered into an amended employment agreement for Ronald P. Erickson which amends our employment agreement with him dated July 1, 2017. The initial one-year term of this amended agreement expired March 21, 2019, and automatically extends for additional one (1) year periods unless either party delivers written notice of such party’s intention to terminate the agreement at least ninety (90) days prior to the end of the renewal term. During the years ended September 30, 2021 and 2020, our compensation committee and our board of directors compensated Mr. Erickson with an annual salary of $195,000 from October 1, 2019 to May 1, 2020. From May 1, 2020 to March 31, 2021, the annual compensation was $215,000. From April 1, 2021 to September 30, 2021, the annual compensation was $300,000. The compensation committee and the board of Particle compensated Mr. Erickson with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. Mr. Erickson will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. If our company terminates Mr. Erickson’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Erickson terminates his employment at any time for “good reason” or due to a “disability,” Mr. Erickson will be entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months.
Outstanding Equity Awards at Fiscal Year-End
Our named executive officers have the following outstanding equity awards as of September 30, 2021.
|
|
| Option Awards | |||||||||||||
| Name |
| Number of securities underlying unexercised options (#) exercisable |
|
| Number of securities underlying unexercised options (#) unexercisable |
|
| Option exercise price ($) |
|
Option expiration date | |||||
| Ronald P. Erickson(1) |
|
| - |
|
|
| 1,200,000 |
|
|
| 1.10 |
|
| 11/04/2024 | |
|
|
|
| - |
|
|
| 1,865,675 |
|
|
| 1.53 |
|
| 12/15/2025 | |
|
|
|
| - |
|
|
| 1,865,675 |
|
|
| 1.53 |
|
| 12/15/2025 | |
|
|
|
| 2,000,000 |
|
|
| - |
|
|
| 1.53 |
|
| 12/15/2025 | |
| Phillip A. Bosua(2) |
|
| 1,000,000 |
|
|
| - |
|
|
| 1.28 |
|
| 07/30/2023 | |
|
|
|
| - |
|
|
| 1,200,000 |
|
|
| 1.10 |
|
| 11/04/2024 | |
|
|
|
| - |
|
|
| 2,132,195 |
|
|
| 1.53 |
|
| 12/15/2025 | |
|
|
|
| - |
|
|
| 2,132,200 |
|
|
| 1.53 |
|
| 12/15/2025 | |
(1) On November 4, 2019, we granted a stock option grant to Ronald P. Erickson for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon uplisting to the NASDAQ or NYSE exchanges. On December 15, 2020, we issued two stock option grants to Ronald P. Erickson, one for 1,865,675 shares and one for 1,865,675 shares, at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria. On December 15, 2020, we issued a fully vested warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five-year warrant is exercisable for cash and on a non-cash basis at $1.53 per share.
(2) On July 30, 2018, Mr. Bosua was awarded a stock option grant for 1,000,000 shares of our common stock that was awarded at $1.28 per share. The stock option grant vests quarterly over four years. On November 4, 2019, we granted a stock option grant to Philip A. Bosua for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon FDA approval of the UBAND blood glucose monitor. On December 15, 2020, we issued two stock option grants to Phillip A. Bosua, one for 2,132,195 shares and one for 2,132,200 shares, at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria.
| 48 |
| Table of Contents |
Additional Narrative Disclosure
Retirement Benefits
We have not maintained, and do not currently maintain, a defined benefit pension plan, nonqualified deferred compensation plan or other retirement benefits.
Potential Payments Upon Termination or Change in Control
As described under “—Employment Agreements” above, Messrs. Bosua and Erickson are entitled severance as described in their employment agreements.
Director Compensation
We primarily use stock options grants to incentive compensation to attract and retain qualified candidates to serve on the board. This compensation reflected the financial condition of our Company. In setting director compensation, we consider the significant amount of time that directors expend in fulfilling their duties to our company as well as the skill-level required by our members of the board. During the year ended September 30, 2021, Ronald P. Erickson and Phillip A. Bosua did not receive any compensation for their service as a director. The compensation disclosed in the Summary Compensation Table above represents the total compensation for Mr. Erickson and Mr. Bosua.
Our independent non-employee directors are not compensated in cash. The only compensation generally has been in the form of stock awards. There is no formal stock compensation plan for independent non-employee directors. Our non-employee directors received the following compensation during the year ended September 30, 2021.
| Name |
| Stock Awards ($)(1) |
|
| Option Awards ($)(1) |
|
| Total ($) |
|
|||
| William A. Owens |
|
| 60,000 |
|
|
| 31,392 |
|
|
| 91,392 |
|
| Jon Pepper |
|
| 60,000 |
|
|
| 31,392 |
|
|
| 91,392 |
|
| Ichiro Takesako |
|
| 60,000 |
|
|
| 31,392 |
|
|
| 91,392 |
|
| (1) | On January 15, 2021, we issued each independent director (i) a stock award for 30,000 shares which was valued at $2.00 per share and (ii) a warrant for 20,000 shares which was valued at the black scholes value of $1.570 per share. These amounts reflect the grant date market value computed in accordance with FASB ASC Topic 718. |
Stock Option Program
Stock options are an integral part of our executive compensation program. They are intended to encourage ownership and retention of our common stock by our named executive officers and employees, as well as non-employee members of our board of directors. Through stock options, the objective of aligning employees’ long-term interest with those of stockholders may be met by providing employees with the opportunity to build a meaningful stake in our company.
Our stock option program assists us by:
|
| · | enhancing the link between the creation of stockholder value and long-term executive incentive compensation; |
|
|
|
|
|
| · | providing an opportunity for increased equity ownership by executive officers; and |
|
|
|
|
|
| · | maintaining competitive levels of total compensation. |
| 49 |
| Table of Contents |
Stock option award levels are determined by the compensation committee and vary among participants’ positions within our company. Newly hired executive officers or promoted executive officers are generally awarded stock options, at the discretion of the compensation committee, at the next regularly scheduled compensation committee meeting on or following their hire or promotion date. In addition, such executives are eligible to receive additional stock options on a discretionary basis after performance criteria are achieved.
Options are awarded at the closing price of our common stock on the date of the grant or last trading day prior to the date of the grant. The compensation committee’s policy is not to grant options with an exercise price that is less than the closing price of our common stock on the grant date.
Generally, the majority of the options granted by the compensation committee vest quarterly over a four-year term. Vesting and exercise rights cease upon termination of employment and/or service, except in the case of death (subject to a one-year limitation), disability or retirement. Stock options vest immediately upon termination of employment without cause or an involuntary termination following a change of control. Prior to the exercise of an option, the holder has no rights as a stockholder with respect to the shares subject to such option, including voting rights and the right to receive dividends or dividend equivalents. During the years ended September 30, 2021 and 2020, we issued stock option grants which vest when earned based on certain performance criteria. No stock compensation expense has been recorded for those options with performance milestones.
Our 2021 Equity Incentive Plan
On August 12, 2021, we established the Know Labs, Inc. 2021 Equity Incentive Plan, or the 2021 Plan. The purpose of the 2021 Plan is to grant restricted stock, stock options and other forms of incentive compensation to officers, employees, directors and consultants of our company and our affiliates (together, our company). The following summary briefly describes the principal features of the 2021 Plan and is qualified in its entirety by reference to the full text of the 2021 Plan, which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Awards that may be granted include: (a) Incentive Stock Options, (b) Non-qualified Stock Options, (c) Stock Appreciation Rights, (d) Restricted Awards, (e) Performance Share Awards, and (f) Performance Compensation Awards. These awards offer our officers, employees, consultants and directors the possibility of future value, depending on the long-term price appreciation of our common stock and the award holder’s continuing service with our company. All of the permissible types of awards under the 2021 Plan are described in more detail as follows:
Purposes of 2021 Plan: The purposes of the 2021 Plan are to attract and retain officers, employees and directors for our company and our subsidiaries; motivate them by means of appropriate incentives to achieve long-range goals; provide incentive compensation opportunities; and further align their interests with those of our stockholders through compensation that is based on our common stock .
Administration of the 2021 Plan: The 2021 Plan is administered by our board of directors which has delegated this responsibility to our compensation committee (which we refer to as the plan administrator). Among other things, the plan administrator has the authority to select persons who will receive awards, determine the types of awards and the number of shares to be covered by awards, and to establish the terms, conditions, performance criteria, restrictions and other provisions of awards. The plan administrator has authority to establish, amend and rescind rules and regulations relating to the 2021 Plan.
Eligible Recipients: Persons eligible to receive awards under the 2021 Plan will be those officers, employees, consultants, and directors of our company and our subsidiaries who are selected by the plan administrator.
Shares Available Under the 2021 Plan: 20,000,000 shares of our common stock were originally authorized as the maximum number of shares of our common stock that may be delivered to participants under the 2021 Plan, subject to adjustment for certain corporate changes affecting the shares, such as stock splits. This number was increased to 22,000,000 shares of common stock as of January 1, 2022 as a result of the automatic share reserve increase discussed below. Shares subject to an award under the 2021 Plan for which the award is canceled, forfeited or expires again become available for grants under the 2021 Plan. Shares subject to an award that is settled in cash will not again be made available for grants under the 2021 Plan. As of the date of this prospectus, all shares remain available for issuance under the 2021 Plan. The 2021 Plan also authorizes for issuance the sum of (A) any shares of our common stock that, as of the date of stockholder approval of the 2021 Plan, have been reserved but not issued pursuant to any awards granted under our 2011 Stock Incentive Plan, as amended, or the 2011 Plan, and (B) any shares of our common stock subject to stock options or similar awards granted under the 2011 Plan that, after the date of stockholder approval of the 2021 Plan, expire or otherwise terminate without having been exercised in full and shares of our common stock issued pursuant to awards granted under the 2011 Plan that are forfeited to or repurchased by us, with the maximum number of shares of our common stock to be added to the 2021 Plan pursuant to clause (ii) equal to 14,650,120.
| 50 |
| Table of Contents |
Automatic Share Reserve Increase: Subject to the provisions of Section 14 of the 2021 Plan, the number of shares available for issuance under the 2021 Plan will be increased on the first day of each calendar year beginning January 1, 2022 and ending on and including January 1, 2030 in an amount equal to the least of (i) 2,000,000 shares of our common stock, (ii) four percent (4%) of the outstanding shares of our common stock on the last day of the immediately preceding fiscal year or (iii) such number of shares of our common stock determined by our board of directors; provided, that such determination under clause (iii) will be made no later than the last day of the immediately preceding fiscal year.
Stock Options: Stock options give the option holder the right to acquire from us a designated number of shares of common stock at a purchase price that is fixed upon the grant of the option. The exercise price will not be less than the market price of the common stock on the date of grant. Stock options granted may be either tax-qualified stock options (so-called ”incentive stock options”) or non-qualified stock options.
General. Subject to the provisions of the 2021 Plan, the plan administrator has the authority to determine all grants of stock options. That determination will include: (i) the number of shares subject to any option; (ii) the exercise price per share; (iii) the expiration date of the option; (iv) the manner, time and date of permitted exercise; (v) other restrictions, if any, on the option or the shares underlying the option; and (vi) any other terms and conditions as the plan administrator may determine.
Option Price. The exercise price for stock options will be determined at the time of grant. Normally, the exercise price will not be less than the fair market value on the date of grant. As a matter of tax law, the exercise price for any incentive stock option awarded may not be less than the fair market value of the shares on the date of grant. However, incentive stock option grants to any person owning more than 10% of our voting stock must have an exercise price of not less than 110% of the fair market value on the grant date.
Exercise of Options. An option may be exercised only in accordance with the terms and conditions for the option agreement as established by the plan administrator at the time of the grant. The option must be exercised by notice to us, accompanied by payment of the exercise price. Payments may be made in cash or, at the option of the plan administrator, by actual or constructive delivery of shares of common stock to the holder of the option based upon the fair market value of the shares on the date of exercise.
Expiration or Termination. Options, if not previously exercised, will expire on the expiration date established by the plan administrator at the time of grant. In the case of incentive stock options, such term cannot exceed ten years provided that in the case of holders of more than 10% of our voting stock, such term cannot exceed five years. Options will terminate before their expiration date if the holder’s service with our company or a subsidiary terminates before the expiration date. The option may remain exercisable for specified periods after certain terminations of employment, including terminations as a result of death, disability or retirement, with the precise period during which the option may be exercised to be established by the plan administrator and reflected in the grant evidencing the award.
Incentive and Non-Qualified Options. As described elsewhere in this summary, an incentive stock option is an option that is intended to qualify under certain provisions of the Code, for more favorable tax treatment than applies to non-qualified stock options. Any option that does not qualify as an incentive stock option will be a non-qualified stock option. Under the Code, certain restrictions apply to incentive stock options. For example, the exercise price for incentive stock options may not be less than the fair market value of the shares on the grant date and the term of the option may not exceed ten years. In addition, an incentive stock option may not be transferred, other than by will or the laws of descent and distribution, and is exercisable during the holder’s lifetime only by the holder. In addition, no incentive stock options may be granted to a holder that is first exercisable in a single year if that option, together with all incentive stock options previously granted to the holder that also first become exercisable in that year, relate to shares having an aggregate market value in excess of $100,000, measured at the grant date.
Stock Appreciation Rights: Stock appreciation rights, or SARs, which may be granted alone or in tandem with options, have an economic value similar to that of options. When a SAR for a particular number of shares is exercised, the holder receives a payment equal to the difference between the market price of the shares on the date of exercise and the exercise price of the shares under the SAR. The exercise price for SARs normally is the market price of the shares on the date the SAR is granted. Under the 2021 Plan, holders of SARs may receive this payment — the appreciation value — either in cash or shares of common stock valued at the fair market value on the date of exercise. The form of payment will be determined by us.
| 51 |
| Table of Contents |
Stock Awards: Restricted shares are shares of common stock awarded to participants at no cost. Restricted shares can take the form of awards of restricted stock, which represent issued and outstanding shares of our common stock subject to vesting criteria, or restricted stock units, which represent the right to receive shares of our common stock, subject to satisfaction of the vesting criteria. Those may include requirements for continuous service and/or the achievement of specified performance goals. Restricted shares are forfeitable and non-transferable until the shares vest. The vesting date or dates and other conditions for vesting are established when the shares are awarded.
Cash Awards: A cash award is an award that may be in the form of cash or shares of common stock or a combination, based on the attainment of pre-established performance goals and other conditions, restrictions and contingencies identified by the plan administrator.
Section 162(m) of the Code: Section 162(m) of the Code limits publicly-held companies to an annual deduction for U.S. federal income tax purposes of $1.0 million for compensation paid to each of their principal executive officer or principal financial officer and their three highest compensated executive officers (other than the principal executive officer or principal financial officer) determined at the end of each year, referred to as covered employees.
Performance Criteria: Under the 2021 Plan, one or more performance criteria will be used by the plan administrator in establishing performance goals. Any one or more of the performance criteria may be used on an absolute or relative basis to measure the performance of our company, as the plan administrator may deem appropriate, or as compared to the performance of a group of comparable companies or published or special index that the plan administrator deems appropriate. In determining the actual size of an individual performance compensation award, the plan administrator may reduce or eliminate the amount of the award through the use of negative discretion if, in its sole judgment, such reduction or elimination is appropriate. The plan administrator shall not have the discretion to (i) grant or provide payment in respect of performance compensation awards if the performance goals have not been attained or (ii) increase a performance compensation award above the maximum amount payable under the 2021 Plan.
Other Material Provisions: Awards will be evidenced by a written agreement, in such form as may be approved by the plan administrator. In the event of various changes to the capitalization of our company, such as stock splits, stock dividends and similar re-capitalizations, an appropriate adjustment will be made by the plan administrator to the number of shares covered by outstanding awards or to the exercise price of such awards. The plan administrator is also permitted to include in the written agreement provisions that provide for certain changes in the award in the event of a change of control of our company, including acceleration of vesting. Except as otherwise determined by the plan administrator at the date of grant, awards will not be transferable, other than by will or the laws of descent and distribution. Prior to any award distribution, we are permitted to deduct or withhold amounts sufficient to satisfy any employee withholding tax requirements. Our board also has the authority, at any time, to discontinue the granting of awards. The board also has the authority to alter or amend the 2021 Plan or any outstanding award or may terminate the 2021 Plan as to further grants, provided that no amendment will, without the approval of our stockholders, to the extent that such approval is required by law or the rules of an applicable exchange, increase the number of shares available under the 2021 Plan, change the persons eligible for awards under the 2021 Plan, extend the time within which awards may be made, or amend the provisions of the 2021 Plan related to amendments. No amendment that would adversely affect any outstanding award made under the 2021 Plan can be made without the consent of the holder of such award.
Our 2011 Stock Incentive Plan
On April 29, 2011, our 2011 Stock Incentive Plan, or the 2011 Plan, was approved at the annual stockholder meeting. Most recently, on November 23, 2020, our board of directors increased the number of shares of our common stock available for issuance under the 2011 Plan to 12,750,000. The 2011 Plan terminated on or about April 19, 2021.
| 52 |
| Table of Contents |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transactions with Related Persons
The following includes a summary of transactions since the beginning of our 2019 fiscal year, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last three completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation described under “Executive Compensation” above). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
Transactions with Clayton Struve
On May 3, 2022, the Company approved the Extension of Warrant Agreement with Clayton Struve, extending the exercise dates as follows:
| Warrant No./Class |
| Issue Date |
| No. Warrant Shares |
|
| Exercise Price |
|
| Original Expiration Date |
| Amended Expiration Date | |||
| Clayton A. Struve Warrant |
| 08-14-2017 |
|
| 1,440,000 |
|
| $ | 0.25 |
|
| 08-13-2023 |
| 08-13-2024 | |
| Clayton A. Struve Warrant |
| 12-12-2017 |
|
| 1,200,000 |
|
| $ | 0.25 |
|
| 12-11-2023 |
| 12-11-2024 | |
| Clayton A. Struve Warrant |
| 08-04-2016 |
|
| 1,785,715 |
|
| $ | 0.25 |
|
| 08-04-2023 |
| 08-04-2024 | |
| Clayton A. Struve Warrant |
| 02-28-2018 |
|
| 1,344,000 |
|
| $ | 0.25 |
|
| 02-28-2023 |
| 02-28-2024 | |
On January 28, 2021, Clayton A. Struve exercised warrants on a cashless basis for 889,880 shares of common stock at $0.25 per share, including warrants for 187,500 and 187,500 that were just extended as discussed above. We recorded interest expense of $244,260 during the nine months ended June 30, 2022 related to the extension of the warrants.
Convertible Promissory Notes with Clayton A. Struve
We owe Clayton A. Struve, a significant stockholder, $1,071,000 under convertible promissory or OID notes. We recorded accrued interest of $75,301 and $71,562 as of March 31, 2021 and September 30, 2020, respectively. On December 23, 2020, we signed amendments to the convertible promissory or OID notes, extending the due dates to March 31, 2021. On April 29, 2021, we signed amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2021. On November 8, 2021, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to March 31, 2022. On May 3, 2022, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2022.
Series C and D Preferred Stock and Warrants
On August 5, 2016, the Company closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve, an accredited investor for the purchase of $1,250,000 of preferred stock with a conversion price of $0.70 per share. The preferred stock has a yield of 8% and an ownership blocker of 4.99%. In addition, Mr. Struve received a five-year warrant to acquire 1,785,714 shares of common stock at $0.70 per share. On August 14, 2017, the price of the Series C Stock were adjusted to $0.25 per share pursuant to the documents governing such instruments.
As of March 31, 2021 and September 30, 2020, we have $750,000 of series D preferred stock outstanding with Mr. Struve.
See “Description of Securities” for the terms of our series C convertible preferred stock and series D convertible preferred stock.
| 53 |
| Table of Contents |
Debt Offering
Mr. Struve invested $1,000,000 in our debt offering which closed in May 2019. On March 18, 2020, Mr. Struve received 1,080,000 shares of common stock related to the automatic conversion of the $1,000,000 invested in the debt offering.
Transactions with Ronald P. Erickson
On March 16, 2018, we entered into a Note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 currently owing under the J3E2A2Z Notes was converted to a Convertible Redeemable Promissory Note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z Account Payable was converted into a Convertible Redeemable Promissory Note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of common stock of our company for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. The warrants were valued at $110,545. Because the note is immediately convertible, the warrants and beneficial conversion were expensed as interest.
On October 4, 2019, Ronald P. Erickson voluntarily cancelled a stock option grant for 1,000,000 shares with an exercise price of $3.03 per share. The grant was related to performance and was not vested.
On November 4, 2019, we granted a stock option grant to Ronald P. Erickson for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon uplisting to the NASDAQ or NYSE exchanges.
On January 1, 2020, we issued 100,000 shares of restricted common stock to Ronald P. Erickson. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $1.90 per share, the market price of our common stock, or $190,000.
On June 1, 2020, Mr. Erickson received a salary of $10,000 per month for work on Particle, Inc. This salary was cancelled as of August 15, 2021.
On July 2, 2020, Particle issued a stock option grant for 1,500,000 shares at $0.10 per share to Ronald P. Erickson. The stock option grant vests (i) 33.3% upon issuance; (ii) 33.3% after the first sale; and (iii) 33.4% after one million in sales are achieved. The stock option grant was forfeited as of September 30, 2021.
On December 15, 2020, we issued a fully vested warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five-year warrant is exercisable for cash or non-cash at $1.53 per share and was valued using a Black-Scholes model at $1,811,691.
On December 15, 2020, we issued two stock option grants to Ronald P. Erickson, one for 1,865,675 shares and one for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria.
During the year ended September 30, 2021, we paid $272,500 of salaries to Mr. Erickson that were previously accrued and reported but were deferred.
On April 4, 2022, the Company approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to September 30, 2022.
On December 16, 2021, the Company issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
Mr. Erickson and/or entities with which he is affiliated also have accrued compensation, travel and interest of approximately $274,640 and $421,599 as of June 30, 2022 and September 30, 2021, respectively.
During the nine months ended June 30, 2022, the Company paid $120,000 of salaries to Mr. Erickson that were previously accrued and reported but were deferred.
| 54 |
| Table of Contents |
Transactions with Phillip A. Bosua
On October 4, 2019, Philip A. Bosua voluntarily cancelled a stock option grant for 1,000,000 shares with an exercise price of $3.03 per share. The grants were related to performance and was not vested.
On November 4, 2019, we granted a stock option grant to Philip A. Bosua for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon FDA approval of the UBAND blood glucose monitor.
On January 1, 2020, we issued 150,000 shares of restricted common stock to Phillip A. Bosua. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $1.90 per share, the market price of our common stock, or $285,000.
On June 1, 2020, Mr. Bosua received a salary of $10,000 per month for work on Particle, Inc. This salary was cancelled as of August 15, 2021.
On July 2, 2020, Particle issued a stock option grant for 1,500,000 shares at $0.10 per share to Philip A. Bosua. The stock option grant vests (i) 33.3% upon issuance; (ii) 33.3% after the first sale; and (iii) 33.4% after one million in sales are achieved. The stock option grant was forfeited as of September 30, 2021.
On December 15, 2020, we issued two stock option grants to Phillip A. Bosua, one for 2,132,195 shares and one for 2,132,200 shares at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria.
On March 18, 2021, we approved a $250,000 bonus for Mr. Bosua. The bonus was paid during April 2021.
On December 16, 2021, the Company issued a stock option grant to Phillip A. Bosua for 1,300,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
We established a digital wallet and corporate account at Circle in order to receive the Ethereum and then convert it to cash. We received $2,908,551 of Ethereum and recorded a reduction in value of $169,884 related to the decline in value of the Ethereum. The accounts receivable-related party was $46,146 as of June 30, 2022. As of June 30, 2022, accrued expenses include approximately $436,378 of expenses, primarily sales and use tax and other expenses. As of June 30, 2022 accrued expenses-related party include $799,353 of unpaid compensation that was due and payable to the CEO for the NFT revenue. This unpaid compensation was paid in July 2022 During 2021, approximately $1.3 million of the selling and transactional costs for the digital assets was paid through the CEO’s personal digital asset account including approximately $1.075 million which was paid to a consultant via the transfer of Ethereum.
Stock Issuances and Cancellations to Named Executive Officers and Directors
On November 4, 2019, we granted stock option grants to two directors totaling 105,000 shares with an exercise price of $1.10 per share. The stock option grants expire in five years. The stock option grants vested immediately.
On January 1, 2020, we issued 120,000 shares of restricted common stock to three directors. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $1.90 per share, the market price of the Company’s common stock, or $228,000.
On January 15, 2021, we issued 30,000 shares each to three directors shares at an exercise price of $2.00 per share.
On January 15, 2021, we issued 20,000 warrants to purchase common stock each to three directors shares at $2.00 per share. The warrants expire on January 15, 2026.
Promoters and Certain Control Persons
Ronald P. Erickson, our founder and Chairman, may be deemed a “promoter” as defined by Rule 405 of the Securities Act. For information regarding compensation, including items of value, that have been provided or that may be provided to these individuals, please refer to “Executive Compensation” above.
| 55 |
| Table of Contents |
The following table sets forth certain information with respect to the beneficial ownership of our each class of our voting securities as of the date of this prospectus for (i) each of our named executive officers and directors; (ii) all of our named executive officers and directors as a group; and (iii) each other stockholder known by us to be the beneficial owner of more than 5% of our outstanding voting securities. The following table assumes that the underwriters have not exercised the over-allotment option.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock that such person or any member of such group has the right to acquire within sixty (60) days of the date of this prospectus. For purposes of computing the percentage of outstanding shares of our common stock held by each person or group of persons named above, any shares that such person or persons has the right to acquire within sixty (60) days of the date of this prospectus are deemed to be outstanding for such person, but not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares listed as beneficially owned does not constitute an admission of beneficial ownership by any person.
Unless otherwise indicated below, each beneficial owner named in the table has sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable. The address for each person shown in the table is c/o Know Labs, Inc. 500 Union Street, Suite 810, Seattle Washington, unless otherwise indicated.
| Name of Beneficial Owner |
| Common Stock Beneficially Owned Prior to this Offering(1) |
|
| Common Stock Beneficially Owned After this Offering(2) |
|
||||||||||
|
|
| Shares |
|
| % |
|
| Shares |
|
| % |
|
||||
| Ronald P. Erickson, Chairman (3) |
|
| 10,239,015 |
|
|
| 19.46 | % |
|
| 10,239,015 |
|
|
| 18.22 | % |
| Phillip A. Bosua, CEO and Director (4) |
|
| 4,167,500 |
|
|
| 9.26 | % |
|
| 4,167,500 |
|
|
| 8.57 | % |
| Peter J. Conley, CFO |
|
| 10,000 |
|
| * |
|
|
| 10,000 |
|
| * |
|
||
| William A. Owens, Director (5) |
|
| 901,670 |
|
|
| 2.04 | % |
|
| 901,670 |
|
|
| 1.89 | % |
| Jon Pepper, Director (6) |
|
| 505,500 |
|
|
| 1.15 | % |
|
| 505,500 |
|
|
| 1.06 | % |
| Ichiro Takesako, Director (7) |
|
| 148,500 |
|
| * |
|
|
| 148,500 |
|
| * |
|
||
| All directors and officers as a group (6 persons named above) |
|
| 15,972,185 |
|
|
| 32.28 | % |
|
| 15,972,185 |
|
|
| 30.08 | % |
| Clayton A. Struve (8) |
|
| 19,511,071 |
|
|
| 4.99 | % |
|
| 19,511,071 |
|
|
| 4.99 | % |
| Todd Baszucki (9) |
|
| 3,879,000 |
|
|
| 8.65 | % |
|
| 3,879,000 |
|
|
| 8.01 | % |
| * | Less than 1% |
|
|
|
| (1) | Based on 43,849,281 shares of common stock issued and outstanding as of the date of this prospectus. |
|
|
|
| (2) | Based on 47,449,281 shares of common stock issued and outstanding after this offering. |
|
|
|
| (3) | Consists of (i) 1,483,085 shares of shares of our common stock beneficially owned by Ronald P. Erickson or entities controlled by Mr. Erickson, (ii) 125,000 shares of our common stock issuable upon the exercise of options exercisable within 60 days, (iii) 3,894,666 shares of our common stock issuable upon the exercise of warrants that are exercisable within 60 days, and (iv) 4,736,264 shares of our common stock issuable upon the conversion of convertible debt that is convertible within 60 days. |
|
|
|
| (4) | Consists of (i) 3,005,000 shares of shares of our common stock held directly by Phillip A. Bosua and (ii) 1,162,500 shares of our common stock issuable upon the exercise of options that are exercisable within 60 days. |
|
|
|
| (5) | Consists of (i) 630,420 shares of our common stock held directly by William A Owens and (ii) 271,250 shares of our common stock issuable upon the exercise of warrants that are exercisable within 60 days. |
|
|
|
| (6) | Consists of (i) 388,000 shares of our common stock held directly by Jon Pepper, (ii) 52,500 shares of our common stock issuable upon the exercise of options exercisable within 60 days and (iii) 65,000 shares of our common stock issuable upon the exercise of warrants exercisable within 60 days. |
|
|
|
| (7) | Consists of (i) 56,000 shares of our common stock held directly by Ichiro Takesako, (ii) 52,500 shares of our common stock issuable upon the exercise of options exercisable within 60 days and (iii) 40,000 shares of our common stock issuable upon the exercise of warrants exercisable within 60 days. |
|
|
|
| (8) | Consists of (i) 849,000 shares of our common stock, (ii) 6,269,715 shares of our common stock issuable upon the exercise of warrants, (iii) 5,000,000 shares of our common stock issuable upon the conversion of our series C preferred stock, (iv) 3,108,356 shares of our common stock issuable upon the conversion of our series D preferred stock and (v) 4,284,000 shares of our common stock issuable upon the conversion of convertible notes. All of the warrants, series C preferred stock, series D preferred stock and convertible notes held by Mr. Struve are subject to a 4.99% blocker pursuant to which shares of our common stock may not be issued to the extent that such issuance would cause Mr. Struve to beneficially own more than 4.99% of our common stock. The address of Mr. Struve is 175 West Jackson Blvd., Suite 440, Chicago, IL 60604. |
|
|
|
| (9) | Consists of (i) 2,879,000 shares of our common stock held directly and (ii) 1,000,000 shares of our common stock issuable upon the exercise of warrants. The address for Mr. Baszucki is 519 Loma Alta Road, Carmel, CA 93923. |
We do not currently have any arrangements which if consummated may result in a change of control of our company.
| 56 |
| Table of Contents |
General
Our authorized capital stock currently consists of 205,000,000 shares, consisting of 200,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of “blank check” preferred stock, par value $0.001 per share.
The following description summarizes important terms of the classes of our capital. This summary does not purport to be complete and is qualified in its entirety by the provisions of our articles of incorporation as amended, restated and supplemented to date, or our articles of incorporation, and our second amended and restated bylaws, or our bylaws, which have been filed as exhibits to the registration statement of which this prospectus is a part.
As of the date of this prospectus, there were 43,849,281 shares of common stock and 2,801,719 shares of preferred stock issued and outstanding.
Common Stock
Voting Rights. The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders. Under our articles of incorporation and bylaws, any corporate action to be taken by vote of stockholders other than for election of directors shall be authorized by the affirmative vote of the majority of votes cast. Directors are elected by a plurality of votes. Stockholders do not have cumulative voting rights.
Dividend Rights. Subject to preferences that may be applicable to any then-outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the board of directors out of legally available funds.
Liquidation Rights. In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then-outstanding shares of preferred stock.
Other Rights. Holders of common stock have no preemptive, conversion or subscription rights and there are no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock.
Preferred Stock
Our articles of incorporation authorize our board to issue up to 5,000,000 shares of preferred stock in one or more series, to determine the designations and the powers, preferences and rights and the qualifications, limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights (including the number of votes per share), redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting the series. Our board of directors could, without stockholder approval, issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of common stock and which could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
Series C Convertible Preferred Stock
We are authorized to issue up 1,785,715 shares of our series C convertible preferred stock, or the series C preferred stock, of which 1,785,715 shares have been issued and are currently outstanding.
Ranking. With respect to dividend rights and rights on liquidation, winding up and dissolution, shares of our series C preferred stock rank senior to all shares of our common stock.
Voting Rights. Each holder of series C preferred stock shall have the right to cast one vote per share of our common stock that would be issuable to such holder upon the conversion of all the shares of series C preferred stock and shall be entitled to notice of any stockholders’ meeting in accordance with our bylaws and are entitled to vote upon such matters and in such manner as may be provided by law. The holders of the series C preferred stock and common stock shall vote together as a single class on all matters.
| 57 |
| Table of Contents |
Liquidation Rights. Upon any liquidation, dissolution or winding-up of our company, whether voluntary or involuntary, that is, a Liquidation, after the satisfaction in full of the debts of our company and the payment of any liquidation preference owed to the holders of any securities senior to the series C preferred stock, the holders of our series C preferred stock shall participate pari passu with the holders of our common stock (on an as-converted basis) in the net assets of our company. Neither the consolidation nor merger of our company into or with any other entity or entities nor the consolidation or merger of any entity or entities into our company shall be deemed to be a Liquidation, but the sale, lease or conveyance of all or substantially all our company’s assets shall be deemed a Liquidation.
Dividend Rights. Each outstanding share of series C preferred stock will accrue cumulative dividends at a rate equal to 8.0% per annum of the series C preferred stock stated value, $0.70, subject to adjustment as provided in the series C preferred stock certificate of designations. Dividends will be payable with respect to any shares of series C preferred stock upon any of the following: (a) upon conversion of such shares in accordance with the provisions of the Series C preferred stock certificate of designations and (b) when, as and if otherwise declared by our board of directors. In the event that we shall at any time pay a dividend on our common stock (other than a dividend payable solely in shares of our common stock) or any other class or series of our capital stock, we shall, at the same time, pay to each holder of series C preferred stock a dividend equal to the dividend that would have been payable to such holder if the shares of series C preferred stock held by such holder had been converted into our common stock on the date of determination of holders of our common stock entitled to receive such dividends without regard to the limitations set forth in the series C preferred stock certificate of designations.
Voluntary Conversion Rights. Each holder of any shares of series C preferred stock shall have the right, at its option at any time, to convert any such shares of series C preferred stock into such number of fully paid and nonassessable whole shares of our common stock as is obtained by multiplying the number of shares of series C preferred stock so to be converted by $0.70, the series C preferred stock stated value, and dividing the result by $0.70, that is, the conversion price, as that conversion price may be adjusted in accordance with the terms of the series C preferred stock certificate of designations, which, among other things, provides for a full ratchet anti-dilution adjustment.
Mandatory Conversion Rights. We may also require, upon notice, the conversion of any or all shares of the series C preferred stock into our common stock provided that; (i) the shares of our common stock into which the series C preferred stock would convert, or the series C conversion shares, are eligible to be sold without restriction pursuant to Rule 144 or a registration statement registering the series C conversion shares for resale has been declared effective by the SEC and (ii) our common stock has been approved for listing on the NASDAQ Capital Market or the New York Stock Exchange.
Conversion Limitation: We shall not effect a conversion of the series C preferred stock, whether voluntary or mandatory, and the holder of any shares of series C preferred stock shall not have the right to voluntarily convert its shares of series C preferred stock, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates) would beneficially own in excess of 4.99% of the shares of our common stock outstanding immediately after giving effect to such conversion.
Other Rights. Holders of series C preferred stock have no preemptive or subscription rights and there are no redemption or sinking fund provisions applicable to the series A preferred stock. The rights, preferences and privileges of the holders of series C preferred stock are subject to, and may be adversely affected by, the rights of the holders of shares of any other series of preferred stock.
Series D Convertible Preferred Stock
We are authorized to issue up 1,016,014 shares of our series D convertible preferred stock, or the series D preferred stock, of which 1,016,014 shares have been issued and are currently outstanding.
Ranking. With respect to dividend rights and rights on liquidation, winding up and dissolution, shares of our series D preferred stock rank senior to all shares of our common stock but junior to our shares of series C preferred stock.
Voting Rights. Each holder of series D preferred stock shall have the right to cast one vote per share of our common stock that would be issuable to such holder upon the conversion of all the shares of series D preferred stock and shall be entitled to notice of any stockholders’ meeting in accordance with our bylaws and are entitled to vote upon such matters and in such manner as may be provided by law. The holders of the series D preferred stock and common stock shall vote together as a single class on all matters.
Liquidation Rights. Upon any liquidation, dissolution or winding-up of our company, whether voluntary or involuntary, that is, a Liquidation, after the satisfaction in full of the debts of our company and the payment of any liquidation preference owed to the holders of any securities senior to the series D preferred stock, the holders of our series D preferred stock shall participate in the distribution of the net assets of our company on a basis senior to the holders of our common stock and to the holders of any other series of preferred stock created after the series D preferred stock. Neither the consolidation nor merger of our company into or with any other entity or entities nor the consolidation or merger of any entity or entities into our company shall be deemed to be a Liquidation, but the sale, lease or conveyance of all or substantially all our company’s assets shall be deemed a Liquidation.
| 58 |
| Table of Contents |
Dividend Rights. Each outstanding share of series D preferred stock will accrue cumulative dividends at a rate equal to 8.0% per annum of the series D preferred stock stated value, $0.70, subject to adjustment as provided in the series D preferred stock certificate of designations. Dividends will be payable with respect to any shares of series D preferred stock upon any of the following: (a) upon conversion of such shares in accordance with the provisions of the series D preferred stock certificate of designations and (b) when, as and if otherwise declared by our board of directors. In the event that we shall at any time pay a dividend on our common stock (other than a dividend payable solely in shares of our common stock) or any other class or series of our capital stock, we shall, at the same time, pay to each holder of series D preferred stock a dividend equal to the dividend that would have been payable to such holder if the shares of series D preferred stock held by such holder had been converted into our common stock on the date of determination of holders of our common stock entitled to receive such dividends without regard to the limitations set forth in the series D preferred stock certificate of designations.
Voluntary Conversion Rights. Each holder of any shares of series D preferred stock shall have the right, at its option at any time, to convert any such shares of series D preferred stock into such number of fully paid and nonassessable whole shares of our common stock as is obtained by multiplying the number of shares of series D preferred stock so to be converted by $0.70, the series D preferred stock stated value, and dividing the result by $0.70, that is, the conversion price, as that conversion price may be adjusted in accordance with the terms of the series D preferred stock certificate of designations, which, among other things, provides for a full ratchet anti-dilution adjustment.
Mandatory Conversion Rights. The series D preferred stock will automatically convert upon (i) the listing of our common stock on the Nasdaq, the New York Stock Exchange, or the NYSE American market; (ii) the shares of our common stock into which the series D preferred stock would convert, the series D conversion shares, are eligible to be sold without restriction pursuant to Rule 144 or a registration statement registering the series D conversion shares for resale has been declared effective by the SEC and remains effective at the time of conversion; and (iii) the average Weighted Average Price (as defined in the series D certificate of designations) of our common stock is at least three (3) times the series D conversion price then in effect for 20 consecutive trading days with average daily trading volume during such period, as reported by Bloomberg, equal to or greater than $200,000.
Conversion Limitation: We shall not effect a conversion of the series D preferred stock, whether voluntary or mandatory, and the holder of any shares of series D preferred stock shall not have the right to voluntarily convert its shares of series D preferred stock, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates) would beneficially own in excess of 4.99% of the shares of our common stock outstanding immediately after giving effect to such conversion.
Other Rights. Holders of series D preferred stock have no preemptive or subscription rights and there are no redemption or sinking fund provisions applicable to the series A preferred stock. The rights, preferences and privileges of the holders of series D preferred stock are subject to, and may be adversely affected by, the rights of the holders of shares of any other series of preferred stock.
Series F Preferred Stock
We are authorized to issue up 500 shares of series F preferred stock, none of which has been issued.
Issuing Restrictions. Shares of series F preferred stock may only be issued to and held of record by members of our board of directors as of August 1, 2018, the date the certificate of designations for the series F preferred stock was filed with the Secretary of State of the State of Nevada, or the series F designations filing date.
Voting Rights. For so long as any shares of the series F preferred stock remain issued and outstanding, the holders thereof, voting separately as a class, will not have any right to vote unless and until a Trigger Event occurs (as defined below). Upon a Trigger Event, and for so long as any shares of the series F preferred stock remain issued and outstanding, the holders thereof, voting separately as a class, shall have the right to vote on all shareholder matters at a rate equal to 100,000 shares of our common stock per share of our series F preferred stock. For example, if all 500 shares of our series F preferred stock are outstanding, the vote of the holders of our series F preferred stock will be equal to fifty million (50,000,000) shares of common stock.
| 59 |
| Table of Contents |
Trigger Event. At any time prior to the Explosion Date (defined below), the following events shall constitute a Trigger Event:
|
| (a) | a bid offer is directed to us to purchase our company which is rejected by our board of directors; |
|
|
|
|
|
| (b) | a tender offer to purchase our shareholder’s shares at cost or at a premium to the then-current market price; |
|
|
|
|
|
| (c) | a proxy vote is called by any shareholder with a proposed vote which would result in: (i) the removal or replacement of any of our then-current directors or series F preferred stockholders, or (ii) the acquisition of another entity, the acquisition of our company or substantially all our assets by another entity, or a share exchange or merger with another entity; or |
|
|
|
|
|
| (d) | a single shareholder or group of shareholders suspected to be working in concert acquires more than 23% of our outstanding common stock; or, any current shareholder already owning shares of our common or preferred stock with aggregate voting power in excess of 23% of the outstanding stock, acquires an additional 10% of our outstanding common stock. |
Explosion Date. The series F preferred stock will remain issued and outstanding until the date which is 731 days after the date of issuance of the series F preferred stock by our board of directors to the members of our board of directors entitled to hold the series F preferred stock, which date we refer to as the Explosion Date, unless such date is extended as the result of a Trigger Event extension (as described below). Upon the Explosion Date, the shares of series F preferred stock then outstanding will automatically be cancelled and returned to treasury without any action required by the holders thereof or our B=board of directors.
Trigger Date Extension. Upon the occurrence of a Trigger Event, the Explosion Date will be extended by 183 days. If no Trigger Event has occurred, there shall be no extensions of the Explosion Date.
Amendments to Articles and Bylaws. So long as the series F preferred stock is outstanding, we shall not, without the affirmative vote of the holders of at least 80% of all outstanding shares of series F preferred stock, voting separately as a class (i) amend, alter or repeal any provision of our articles of incorporation or bylaws so as to adversely affect the designations, preferences, limitations and relative rights of the series F preferred stock or (ii) effect any reclassification of the series F preferred stock.
Amendment of Rights of Series F Preferred Stock. We may not, without the affirmative vote of the holders of at least a majority of all outstanding shares of the series F preferred stock, amend, alter or repeal any provision of the series F preferred stock certificate of designations, provided, however, that we may, by any means authorized by law and without any vote of the holders of shares of the Series F Preferred Stock, make technical, corrective, administrative or similar changes to the series F preferred stock certificate of designations that do not, individually or in the aggregate, adversely affect the rights or preferences of the holders of shares of the series F preferred stock.
Liquidation Rights. The holders of our series F preferred stock will not be entitled to any liquidation preference.
Dividend Rights. The holders of our series F preferred stock will not be entitled to any dividends.
Convertibility. The shares of our series F preferred stock do not have any conversion rights.
Redemption Rights. The series F preferred stock is not redeemable.
Transferability. Upon a termination or resignation from our board of directors of any holder of our series F preferred stock, the shares of our series F preferred stock held by such person will automatically be transferred and assigned pro-rata to the remaining members of our board of directors then eligible to hold shares of our series F preferred stock
without any consent or approval being required from the holder.
| 60 |
| Table of Contents |
Protective Provisions. Subject to the rights of other series of our preferred stock which may from time to time come into existence, so long as any shares of series F preferred stock are outstanding, we shall not without first obtaining the approval (by written consent, as provided by law) of the holders of at least 80% of the then outstanding shares of series F preferred stock, voting together as a class, except as otherwise provided for in the series F preferred stock certificate of designations:
|
| (a) | increase or decrease the total number of authorized shares of series F preferred stock; |
|
|
|
|
|
| (b) | effect an exchange, reclassification, or cancellation of all or a part of the series F preferred stock, excluding a reverse stock split or forward stock split; |
|
|
|
|
|
| (c) | effect an exchange, or create a right of exchange, of all or part of the shares of another class of shares into shares of series F preferred stock; or |
|
|
|
|
|
| (d) | Alter or change the rights, preferences or privileges of the shares of series F preferred stock so as to affect adversely the shares of such series, including the rights set forth in the series F preferred stock certificate of Designations. |
Representative’s Warrants
Upon the closing of this offering, there will be up to 289,800 shares of common stock issuable upon exercise of the representative’s warrants. See “Underwriting” below.
Warrants
As of the date of this prospectus, we have issued warrants for the purchase of 21,644,013 shares of common stock at a weighted average price of $1.004. The expiration dates of these warrants range from now to July 7, 2027.
In the future, if we sell our common stock at a price below $0.25 per share, the exercise price of additional outstanding warrants to purchase 10,274,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments. Warrants totaling 4,487,207 would adjust below $1.20 per share pursuant to the documents governing such instruments. Warrants totaling 3,954,625 would adjust below $2.40 per share pursuant to the documents governing such instruments.
Clayton Struve has warrants to purchase 6,269,715 shares of common stock that have a beneficial ownership blocker at 4.99%.
The proceeds of warrants currently outstanding, which are not expected to be exercised on a cashless basis, may generate potential proceeds of up to $16,366,000. We cannot provide assurance that any of these warrants will be exercised.
Options
As of the date of this prospectus, we have issued stock options for the purchase of 21,067,370 shares of common stock at a weighted average price of $1.632. The expiration dates of these stock options range from now to July 6, 2027. There are unearned stock option grants totaling 11,550,745 shares related to performance targets.
Convertible Promissory Notes
We owe Clayton A. Struve $1,071,000 under convertible promissory or OID notes. Our company recorded accrued interest of $84,671 and $79,062 as of June 30, 2022 and September 30, 2021, respectively. On May 3, 2022, our company signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2022.
On March 16, 2018, we entered into a Note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 then owing under the J3E2A2Z Notes was converted to a convertible redeemable promissory note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z account payable was converted into a convertible redeemable promissory note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of our common stock for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. The warrants were valued at $110,545. Because the note is immediately convertible, the warrants and beneficial conversion were expensed as interest. We recorded accrued interest of $269,383 and $216,246 as of June 30, 2022 and September 30, 2021, respectively. On April 4, 2022, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to September 30, 2022.
| 61 |
| Table of Contents |
Anti-Takeover Provisions
Provisions of the Nevada Revised Statutes, our articles of incorporation and our bylaws could have the effect of delaying or preventing a third-party from acquiring us, even if the acquisition would benefit our stockholders. Such provisions of the Nevada Revised Statutes, our articles of incorporation and our bylaws are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by the board of directors and to discourage certain types of transactions that may involve an actual or threatened change of control of our company. These provisions are designed to reduce our vulnerability to an unsolicited proposal for a takeover that does not contemplate the acquisition of all of our outstanding shares, or an unsolicited proposal for the restructuring or sale of all or part of our company.
Nevada Anti-Takeover Statutes
Pursuant to our articles of incorporation, we have elected not to be governed by the terms and provisions of Nevada’s control share acquisition laws (Nevada Revised Statutes 78.378 - 78.3793), which prohibit an acquirer, under certain circumstances, from voting shares of a corporation’s stock after crossing specific threshold ownership percentages, unless the acquirer obtains the approval of the issuing corporation’s stockholders. The first such threshold is the acquisition of at least one-fifth but less than one-third of the outstanding voting power.
Pursuant to our articles of incorporation, we have also elected not to be governed by the terms and provisions of Nevada’s combination with interested stockholders statute (Nevada Revised Statutes 78.411 - 78.444) which prohibits an “interested stockholder” from entering into a “combination” with the corporation, unless certain conditions are met. An “interested stockholder” is a person who, together with affiliates and associates, beneficially owns (or within the prior two years, did beneficially own) 10 percent or more of the corporation’s voting stock, or otherwise has the ability to influence or control such corporation’s management or policies.
Bylaws
In addition, various provisions of our bylaws may also have an anti-takeover effect. These provisions may delay, defer or prevent a tender offer or takeover attempt of the company that a stockholder might consider in his or her best interest, including attempts that might result in a premium over the market price for the shares held by our stockholders. Our bylaws may be adopted, amended or repealed by the affirmative vote of the holders of at least a majority of our outstanding shares of capital stock entitled to vote for the election of directors, and except as provided by Nevada law, our board of directors shall have the power to adopt, amend or repeal the bylaws by a vote of not less than a majority of our directors. Any bylaw provision adopted by the board of directors may be amended or repealed by the holders of a majority of the outstanding shares of capital stock entitled to vote for the election of directors. Our bylaws also contain limitations as to who may call special meetings as well as require advance notice of stockholder matters to be brought at a meeting. Additionally, our bylaws also provide that no director may be removed by less than a two-thirds vote of the issued and outstanding shares entitled to vote on the removal. Our bylaws also permit the board of directors to establish the number of directors and fill any vacancies and newly created directorships. These provisions will prevent a stockholder from increasing the size of our board of directors and gaining control of our board of directors by filling the resulting vacancies with its own nominees.
Our bylaws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to the board of directors. Stockholders at an annual meeting will only be able to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of the board of directors or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given us timely written notice, in proper form, of the stockholder’s intention to bring that business before the meeting. Although our bylaws do not give the board of directors the power to approve or disapprove stockholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting, our bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of our company.
Authorized but Unissued Shares
Our authorized but unissued shares of common stock are available for our board of directors to issue without stockholder approval. We may use these additional shares for a variety of corporate purposes, including raising additional capital, corporate acquisitions and employee stock plans. The existence of our authorized but unissued shares of common stock could render it more difficult or discourage an attempt to obtain control of the company by means of a proxy context, tender offer, merger or other transaction since our board of directors can issue large amounts of capital stock as part of a defense to a take-over challenge. In addition, we have authorized in our articles of incorporation 5,000,000 shares of preferred stock, of which 1,785,715 shares of series C convertible preferred stock and 1,016,014 shares of series D convertible preferred stock are currently designated and outstanding. Our board of directors, acting alone and without approval of our stockholders, can designate and issue one or more series of preferred stock containing super-voting provisions, enhanced economic rights, rights to elect directors, or other dilutive features, that could be utilized as part of a defense to a take-over challenge. We have designated 500 shares of series F preferred stock that contain super-majority voting rights, No shares of our designated series F preferred stock are currently outstanding.
| 62 |
| Table of Contents |
Supermajority Voting Provisions
Nevada Law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s articles of incorporation or bylaws, unless a corporation’s articles of incorporation or bylaws, as the case may be, require a greater percentage. Although our articles of incorporation and bylaws do not currently provide for such a supermajority vote on any matters, our board of directors can amend our bylaws and we can, with the approval of our stockholders, amend our articles of incorporation to provide for such a super-majority voting provision.
Cumulative Voting
Furthermore, neither the holders of our common stock nor the holders of our preferred stock have cumulative voting rights in the election of our directors. The combination of the present ownership by a few stockholders of a significant portion of our issued and outstanding common stock and lack of cumulative voting makes it more difficult for other stockholders to replace our board of directors or for a third party to obtain control of our company by replacing our board of directors.
Listing
We plan to file an application to list our common stock on NYSE American under the symbol “KNW.”
Transfer Agent and Registrar
We have appointed American Stock Transfer & Trust Company located at 6201 15th Avenue, Brooklyn, New York 11219, telephone number is (800) 937-5449, as the transfer agent for our common stock.
| 63 |
| Table of Contents |
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of shares of our common stock, including shares issued upon the conversion of convertible notes, the exercise of outstanding options and warrants, in the public market after this offering, or the possibility of these sales occurring, could cause the prevailing market price for our common stock to fall or impair our ability to raise equity capital in the future.
Immediately following the closing of this offering, we will have 47,449,281 shares of common stock issued and outstanding. In the event the Underwriter exercises the over-allotment option to purchase additional shares of common stock in full, we will have 47,989,281 shares of common stock issued and outstanding. The common stock sold in this offering will be freely tradable without restriction or further registration or qualification under the Securities Act.
Previously issued shares of common stock that were not offered and sold in this offering, as well as shares issuable upon the exercise of warrants and subject to employee stock options, are or will be upon issuance, “restricted securities,” as that term is defined in Rule 144 under the Securities Act. These restricted securities are eligible for public sale only if such public resale is registered under the Securities Act or if the resale qualifies for an exemption from registration under Rule 144 or Rule 701 under the Securities Act, which are summarized below.
Rule 144
In general, a person who has beneficially owned restricted shares of our common stock for at least twelve months, or at least six months in the event we have been a reporting company under the Exchange Act for at least ninety (90) days before the sale, would be entitled to sell such securities, provided that such person is not deemed to be an affiliate of ours at the time of sale or to have been an affiliate of ours at any time during the ninety (90) days preceding the sale. A person who is an affiliate of ours at such time would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of shares that does not exceed the greater of the following:
|
| · | 1% of the number of shares of our common stock then outstanding; or |
|
|
|
|
|
| · | 1% of the average weekly trading volume of our common stock during the four calendar weeks preceding the filing by such person of a notice on Form 144 with respect to the sale; |
provided that, in each case, we are subject to the periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Rule 144 trades must also comply with the manner of sale, notice and other provisions of Rule 144, to the extent applicable.
Rule 701
In general, Rule 701 allows a stockholder who purchased shares of our capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of ours during the immediately preceding 90 days to sell those shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. All holders of Rule 701 shares, however, are required to wait until ninety (90) days after the date of this prospectus before selling shares pursuant to Rule 701.
Lock-Up Agreements
We, and all of our directors and officers have agreed with the underwriters, subject to certain exceptions, not to sell, transfer or dispose of, directly or indirectly, any of our common stock or securities convertible into or exercisable or exchangeable for our common stock for a period of six months after the closing of this offering. See “Underwriting.”
| 64 |
| Table of Contents |
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S.
HOLDERS OF OUR COMMON STOCK
The following is a summary of the material U.S. federal income and estate tax consequences of the ownership and disposition of our common stock that is being issued pursuant to this offering. This summary is limited to Non-U.S. Holders (as defined below) that hold our common stock as a capital asset (generally, property held for investment) for U.S. federal income tax purposes. This summary does not discuss all of the aspects of U.S. federal income and estate taxation that may be relevant to a Non-U.S. Holder in light of the Non-U.S. Holder’s particular investment or other circumstances. Accordingly, all prospective Non-U.S. Holders should consult their own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the ownership and disposition of our common stock.
This summary is based on provisions of the Code, applicable U.S. Treasury regulations and administrative and judicial interpretations, all as in effect or in existence on the date of this prospectus. Subsequent developments in U.S. federal income or estate tax law, including changes in law or differing interpretations, which may be applied retroactively, could alter the U.S. federal income and estate tax consequences of owning and disposing of our common stock as described in this summary. There can be no assurance that the Internal Revenue Service, or IRS, will not take a contrary position with respect to one or more of the tax consequences described herein and we have not obtained, nor do we intend to obtain, a ruling from the IRS with respect to the U.S. federal income or estate tax consequences of the ownership or disposition of our common stock.
As used in this summary, the term “Non-U.S. Holder” means a beneficial owner of our common stock that is not, for U.S. federal income tax purposes:
|
| · | an individual who is a citizen or resident of the United States; |
|
|
|
|
|
| · | a corporation (or other entity treated as a corporation) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
|
|
|
|
|
| · | an entity or arrangement treated as a partnership; |
|
|
|
|
|
| · | an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or |
|
|
|
|
|
| · | a trust, if (1) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more “United States persons” (within the meaning of the Code) has the authority to control all of the trust’s substantial decisions, or (2) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a United States person. |
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in such a partnership generally will depend upon the status of the partner, the activities of the partnership and certain determinations made at the partner level. Partnerships, and partners in partnerships, that hold our common stock should consult their own tax advisors as to the particular U.S. federal income and estate tax consequences of owning and disposing of our common stock that are applicable to them.
This summary does not consider any specific facts or circumstances that may apply to a Non-U.S. Holder and does not address any special tax rules that may apply to particular Non-U.S. Holders, such as:
|
| · | a Non-U.S. Holder that is a financial institution, insurance company, tax-exempt organization, pension plan, broker, dealer or trader in securities, dealer in currencies, U.S. expatriate, controlled foreign corporation or passive foreign investment company; |
|
|
|
|
|
| · | a Non-U.S. Holder holding our common stock as part of a conversion, constructive sale, wash sale or other integrated transaction or a hedge, straddle or synthetic security; |
|
|
|
|
|
| · | a Non-U.S. Holder that holds or receives our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; or |
|
|
|
|
|
| · | a Non-U.S. Holder that at any time owns, directly, indirectly or constructively, 5% or more of our outstanding common stock. |
| 65 |
| Table of Contents |
In addition, this summary does not address any U.S. state or local, or non-U.S. or other tax consequences, or any U.S. federal income or estate tax consequences for beneficial owners of a Non-U.S. Holder, including stockholders of a controlled foreign corporation or passive foreign investment company that holds our common stock.
Each Non-U.S. Holder should consult its own tax advisor regarding the U.S. federal, state, local and non-U.S. income and other tax consequences of owning and disposing of our common stock.
Distributions on Our Common Stock
We do not currently expect to pay any cash dividends on our common stock. If we make distributions of cash or property (other than certain pro rata distributions of our common stock) with respect to our common stock, any such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax rules. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a nontaxable return of capital to the extent of the Non-U.S. Holder’s adjusted tax basis in our common stock and will reduce (but not below zero) such Non-U.S. Holder’s adjusted tax basis in our common stock. Any remaining excess will be treated as gain from a disposition of our common stock subject to the tax treatment described below in “— Dispositions of Our common stock .”
Distributions on our common stock that are treated as dividends and that are effectively connected with a Non-U.S. Holder’s conduct of a trade or business in the United States will be taxed on a net income basis at the regular graduated rates and in the manner applicable to United States persons. An exception may apply if the Non-U.S. Holder is eligible for, and properly claims, the benefit of an applicable income tax treaty and the dividends are not attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States. In such case, the Non-U.S. Holder may be eligible for a lower rate under an applicable income tax treaty between the United States and its jurisdiction of tax residence. Dividends that are effectively connected with a Non-U.S. Holder’s conduct of a trade or business in the United States will not be subject to the U.S. withholding tax if the Non-U.S. Holder provides to the applicable withholding agent a properly executed IRS Form W-8ECI (or other applicable form) in accordance with the applicable certification and disclosure requirements. A Non-U.S. Holder treated as a corporation for U.S. federal income tax purposes may also be subject to a “branch profits tax” at a 30% rate (unless the Non-U.S. Holder is eligible for a lower rate under an applicable income tax treaty) on the Non-U.S. Holder’s earnings and profits (attributable to dividends on our common stock or otherwise) that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States. The amount of taxable earnings and profits is generally reduced by amounts reinvested in the operations of the U.S. trade or business and increased by any decline in its equity.
The certifications described above must be provided to the applicable withholding agent prior to the payment of dividends and must be updated periodically. A Non-U.S. Holder may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS. Non-U.S. Holders should consult their own tax advisors regarding their eligibility for benefits under any relevant income tax treaty and the manner of claiming such benefits.
The foregoing discussion is subject to the discussions below under “—Backup Withholding and Information Reporting” and “—FATCA Withholding.”
Dispositions of Our Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax (including U.S. withholding tax) on gain recognized on any sale or other disposition of our common stock unless:
|
| · | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States); in such case, the gain would be subject to U.S. federal income tax on a net income basis at the regular graduated rates and in the manner applicable to United States persons (unless an applicable income tax treaty provides otherwise) and, if the Non-U.S. Holder is treated as a corporation for U.S. federal income tax purposes, the “branch profits tax” described above may also apply; |
|
|
|
|
|
| · | the Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of the disposition and meets certain other requirements; in such case, except as otherwise provided by an applicable income tax treaty, the gain, which may be offset by certain U.S. source capital losses, generally will be subject to a flat 30% U.S. federal income tax, even if the Non-U.S. Holder is not treated as a resident of the United States under the Code; or |
|
|
|
|
|
| · | we are or have been a “United States real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of (i) the five-year period ending on the date of disposition and (ii) the period that the Non-U.S. Holder held our common stock. |
| 66 |
| Table of Contents |
Generally, a corporation is a “United States real property holding corporation” if the fair market value of its “United States real property interests” equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. We believe that we are not currently, and we do not anticipate becoming in the future, a United States real property holding corporation. However, because the determination of whether we are a United States real property holding corporation is made from time to time and depends on the relative fair market values of our assets, there can be no assurance in this regard. If we were a United States real property holding corporation, the tax relating to disposition of stock in a United States real property holding corporation generally will not apply to a Non-U.S. Holder whose holdings, direct, indirect and constructive, constituted 5% or less of our common stock at all times during the applicable period, provided that our common stock are “regularly traded on an established securities market” (as provided in applicable U.S. Treasury regulations) at any time during the calendar year in which the disposition occurs. However, no assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. Non-U.S. Holders should consult their own tax advisors regarding any possible adverse U.S. federal income tax consequences to them if we are, or were to become, a United States real property holding corporation.
The foregoing discussion is subject to the discussions below under “—Backup Withholding and Information Reporting” and “—FATCA Withholding.”
Federal Estate Tax
Any shares of our common stock that are owned (or treated as owned) by an individual who is not a U.S. citizen or resident of the United States (as specially defined for U.S. federal estate tax purposes) at the time of death will be included in that individual’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax or other treaty provides otherwise and, therefore, may be subject to U.S. federal estate tax.
Backup Withholding and Information Reporting
Backup withholding (currently at a rate of 24%) may apply to dividends paid by U.S. corporations in some circumstances, but will not apply to payments of dividends on our common stock to a Non-U.S. Holder if the Non-U.S. Holder provides to the applicable withholding agent a properly executed IRS Form W-8BEN or W-8BEN-E (or other applicable form) certifying under penalties of perjury that the Non-U.S. Holder is not a United States person or is otherwise entitled to an exemption. However, the applicable withholding agent generally will be required to report to the IRS (and to such Non-U.S. Holder) payments of dividends on our common stock and the amount of U.S. federal income tax, if any, withheld from those payments. In accordance with applicable treaties or agreements, the IRS may provide copies of such information returns to the tax authorities in the country in which the Non-U.S. Holder resides.
The gross proceeds from sales or other dispositions of our common stock may be subject, in certain circumstances discussed below, to U.S. backup withholding and information reporting. If a Non-U.S. Holder sells or otherwise disposes of any of our common stock outside the United States through a non-U.S. office of a non-U.S. broker and the disposition proceeds are paid to the Non-U.S. Holder outside the United States, the U.S. backup withholding and information reporting requirements generally will not apply to that payment. However, U.S. information reporting, but not U.S. backup withholding, will apply to a payment of disposition proceeds, even if that payment is made outside the United States, if a Non-U.S. Holder sells our common stock through a non-U.S. office of a broker that is a United States person or has certain enumerated connections with the United States, unless the broker has documentary evidence in its files that the Non-U.S. Holder is not a United States person and certain other conditions are met or the Non-U.S. Holder otherwise qualifies for an exemption.
If a Non-U.S. Holder receives payments of the proceeds of a disposition of our common stock to or through a U.S. office of a broker, the payment will be subject to both U.S. backup withholding and information reporting unless the Non-U.S. Holder provides to the broker a properly executed IRS Form W-8BEN or W-8BEN-E (or other applicable form) certifying under penalties of perjury that the Non-U.S. Holder is not a United States person, or the Non-U.S. Holder otherwise qualifies for an exemption.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be credited against the Non-U.S. Holder’s U.S. federal income tax liability (which may result in the Non-U.S. Holder being entitled to a refund), provided that the required information is timely furnished to the IRS.
FATCA Withholding
The Foreign Account Tax Compliance Act and related Treasury guidance (commonly referred to as FATCA) impose U.S. federal withholding tax at a rate of 30% on payments to certain foreign entities of (i) U.S.-source dividends (including dividends paid on our common stock) and (ii) the gross proceeds from the sale or other disposition of property that produces U.S.-source dividends (including sales or other dispositions of our common stock). This withholding tax applies to a foreign entity, whether acting as a beneficial owner or an intermediary, unless such foreign entity complies with (i) certain information reporting requirements regarding its U.S. account holders and its U.S. owners and (ii) certain withholding obligations regarding certain payments to its account holders and certain other persons. Accordingly, the entity through which a Non-U.S. Holder holds its common stock will affect the determination of whether such withholding is required. While withholding under FATCA would have also applied to payments of gross proceeds from the sale or other disposition of our common stock on or after January 1, 2019, U.S. Treasury regulations proposed in December, 2018 eliminate such withholding on payments of gross proceeds entirely. Taxpayers generally may rely on these proposed U.S. Treasury regulations until final U.S. Treasury regulations are issued. Non-U.S. Holders are encouraged to consult their tax advisors regarding FATCA.
| 67 |
| Table of Contents |
In connection with this offering, we expect to enter an underwriting agreement with Boustead Securities, LLC, as representative of the underwriters named in this prospectus, with respect to the common stock in this offering. Under the terms and subject to the conditions contained in the underwriting agreement, the representative will agree to purchase from us on a firm commitment basis the respective number of shares at the public price less the underwriting discounts and commissions set forth on the cover page of this prospectus, and each of the underwriters has severally agreed to purchase, and we have agreed to sell to the underwriters, at the public offering price per shares less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares listed next to its name in the following table.
| Underwriter |
| Number of Shares |
|
|
| Boustead Securities, LLC |
|
| 3,600,000 |
|
| Total |
|
| 3,600,000 |
|
The common stock sold by the underwriters to the public will initially be offered at the public offering price set forth on the cover page of this prospectus. Any common stock sold by the underwriters to securities dealers may be sold at a discount from the public offering price not to exceed $0.08 per share. If all of the shares are not sold at the initial offering price, the representative may change the offering price and the other selling terms. The representative has advised us that the underwriters do not intend to make sales to discretionary accounts. The underwriting agreement will provide that the obligations of the underwriters to pay for and accept delivery of the shares are subject to the passing upon certain legal matters by counsel and certain conditions such as confirmation of the accuracy of representations and warranties by us about our financial condition and operations and other matters. The obligation of the underwriters to purchase the shares is conditioned upon our receiving approval to list our common stock on NYSE American.
Over-Allotment Option
If the underwriters sell more shares than the total number set forth in the table above, we have granted to the underwriters an option, exercisable one or more times in whole or in part, not later than 45 days after the date of this prospectus, to purchase up to 540,000 additional shares at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, constituting 15% of the total number of shares to be offered in this offering (excluding shares subject to this option). The underwriters may exercise this option solely for the purpose of covering over-allotments in connection with this offering. This offering is being conducted on a firm commitment basis. Any shares issued or sold under the option will be issued and sold on the same terms and conditions as the other shares that are the subject of this offering.
Discounts and Commissions; Expenses
The underwriting discounts and commissions are a cash fee equal to 7% of gross proceeds from the sale of common stock in this offering. We have been advised by the representative that the underwriters propose to offer the common stock to the public at the public offering price set forth on the cover of this prospectus and to dealers at a price that represents a concession not in excess of $0.08 per share under the public offering price. After the offering, the representative may change the public offering price and other selling terms.
The following table summarizes the public offering price and the underwriting discounts and commissions payable to the underwriters by us in connection with this offering:
|
|
| Per Share |
|
| Without Over-Allotment Option |
|
| With Over-Allotment Option |
|
|||
| Public offering price |
| $ | 2.00 |
|
| $ | 7,200,000 |
|
| $ | 8,280,000 |
|
| Underwriting discounts and commissions (7%) |
|
| 0.14 |
|
|
| 504,000 |
|
|
| 579,600 |
|
| Non-accountable expense allowance (1.00%) |
|
| 0.02 |
|
|
| 72,000 |
|
|
| 82,800 |
|
| Proceeds to us, before expenses |
| $ | 1.84 |
|
| $ | 6,624,000 |
|
| $ | 7,617,600 |
|
We have agreed to pay the representative a non-accountable expense allowance equal to 1.00% of the gross proceeds received at the closing of this offering.
We have agreed to reimburse the representative for reasonable out-of-pocket expenses incurred by it in connection with this offering, regardless of whether the offering is consummated, up to approximately $117,500. The out of pocket expenses include but are not limited to: (i) reasonable fees of representative’s legal counsel, up to $75,000, (ii) the cost of background check on our officers, directors and major shareholders, up to $2,500; and (iii) up to $40,000 in due diligence expenses. Any out-of-pocket expenses other than those referenced in the previous sentence above $5,000 are to be pre-approved by our company. As of the date of this prospectus, we have paid the representative refundable advances of $25,000 which shall be applied against its actual out-of-pocket accountable expenses. Such advance payments will be returned to us to the extent any portion of the advance is not actually incurred, in accordance with FINRA Rule 5110(g)(4)(A).
| 68 |
| Table of Contents |
We estimate that our total expenses of the offering, excluding the estimated underwriting discounts and commissions and the non-accountable expense allowance, will be approximately $277,000.
Representative’s Warrants
We have agreed to issue to the representative (or its permitted assignees) warrants to purchase up to a total of 252,000 shares of common stock, or 289,800 shares if the underwriters exercise the over-allotment option in full (equal to 7% of the aggregate number of the shares sold in this offering), at an exercise price of $2.40 (equal to 120% of the public offering price of the common stock sold in this offering). The representative’s warrants will be exercisable at any time, and from time to time, in whole or in part, commencing from the closing of the offering and expiring five (5) years from the commencement of sales in the offering and will have a cashless exercise provision. The representative’s warrants are not exercisable or convertible for more than five years from the commencement of sales of the public offering. The representative’s warrants will also provide for customary anti-dilution provisions and immediate piggyback registration rights with respect to the registration of the shares underlying the warrants for a period of five years from commencement of sales of this offering. The warrants are not redeemable by us. The representative’s warrants and the shares of common stock issuable upon exercise of the representative’s warrants have been included on the registration statement of which this prospectus forms a part.
The representative’s warrants and the underlying shares are deemed to be compensation by FINRA, and therefore will be subject to a 180-day lock-up period pursuant to FINRA Rule 5110(e)(1). In accordance with FINRA Rule 5110(e)(1), neither the representative’s warrants nor any of our common stock issued upon exercise of the representative’s warrants may be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of such securities by any person, for a period of 180 days immediately following commencement of sale of this offering subject to certain exceptions permitted by FINRA Rule 5110(e)(2).
Indemnification
We have agreed to indemnify the representative and the other underwriters, if any, against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to payments that the representative and the other underwriters may be required to make for these liabilities.
Right of First Refusal
We have agreed to provide the representative the right of first refusal for two (2) years following the consummation of this offering, provided that in no event shall the duration of this right extend past the three-year anniversary of the commencement date of sales in this offering, to act as financial advisor on any public or private financing (debt or equity), merger, business combination, recapitalization or sale of some or all of our equity or our assets. In the event that we engage the representative to provide such services, the representative will be compensated consistent with our engagement agreement with the representative, unless we mutually agree otherwise.
No Sales of Similar Securities
We have agreed not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of common stock at a price per share that is less than the price per shares of common stock in this offering, or modify the terms of any existing securities, whether in conjunction with another broker-dealer or on our company’s own volition for a period of twelve months following date on which our common stock is trading on NYSE American, without the prior written consent of the representative.
Lock-Up Agreements
Our executive officers and directors following this offering have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any shares of our common stock for a period of six months following the closing of this offering, subject to certain exceptions.
| 69 |
| Table of Contents |
Notwithstanding the above, the representative of this offering may engage in stabilization activities as described below. The representative may in its sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the lock-up period. When determining whether or not to release shares from the lock-up agreements, the representative will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act:
|
| · | Stabilizing transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum. |
|
|
|
|
|
| · | Over-allotment transactions involve sales by the underwriters of securities in excess of the number of securities the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriters is not greater than the number of securities that they may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriters may close out any covered short position by either exercising its over-allotment option and/or purchasing securities in the open market. |
|
|
|
|
|
| · | Syndicate covering transactions involve purchases of the securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared to the price at which they may purchase securities through the over-allotment option. A naked short position occurs if the underwriters sells more securities than could be covered by the over-allotment option. This position can only be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in this offering. |
|
|
|
|
|
| · | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when securities originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of the securities. As a result, the price of our shares of common stock and warrants may be higher than the price that might otherwise exist in the open market. These transactions may be discontinued at any time.
Determination of Offering Price
In determining the public offering price, we and the representative have considered a number of factors, including:
|
| · | the information set forth in this prospectus and otherwise available to the representative; |
|
|
|
|
|
| · | our prospects and the history and prospects for the industry in which we compete; |
|
|
|
|
|
| · | an assessment of our management; |
|
|
|
|
|
| · | our prospects for future revenues and earnings; |
|
|
|
|
|
| · | the general condition of the securities markets at the time of this offering; |
|
|
|
|
|
| · | the recent market prices of, and demand for, publicly traded securities of generally comparable companies, as well as the recent market price of our common stock; and |
|
|
|
|
|
| · | other factors deemed relevant by the representative and us. |
| 70 |
| Table of Contents |
The estimated public offering price set forth on the cover page of this preliminary prospectus is subject to change as a result of market conditions and other factors. Neither we nor the representative can assure investors that an active trading market will develop for our common stock, or that the shares will trade in the public market at or above the public offering price.
Electronic Offer, Sale and Distribution of Common Stock
A prospectus in electronic format may be delivered to potential investors by the underwriters in this offering. In addition, shares of common stock may be sold by the underwriters to securities dealers who resell our common stock to online brokerage account holders. The prospectus in electronic format will be identical to the paper version of such prospectus. Other than the prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the representative in its capacity as representative and should not be relied upon by investors.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Other
The representative provided us with placement agent services pursuant to a placement agent agreement entered into on November 6, 2018, pursuant to which the representative received the following fees: (i) cash commissions of $1,443,911 and expense reimbursements of $18,046 paid from November 2018 until September 2021; (ii) private placement warrants to purchase (a) 1,040,774 shares of common stock at an exercise price of $1.20 issued between March 2019 and June 2020; and (b) 532,671 shares of common stock at an exercise price of $2.40 issued between March 2021 and August 2021; and (iii) $25,000 in advisory fees.
The representative entered into an advisory agreement with us on September 27, 2021 to provide advisory and placement services to our wholly owned subsidiary, Particle, pursuant to which the representative received; (i) cash commissions of $76,208 during the period from February 2021 until August 2021; and (ii) $10,000 in advisory fees. On May 31, 2022, Mr. Conley purchased 10,000 shares of common stock in an open market transaction at an average purchase price of $1.512 per share. The shares are deemed to be compensation by FINRA, and therefore will be subject to a 180 day lockup period pursuant to FINRA Rule 5110(e)(1). In accordance with FINRA Rule 5110(e)(1), the common stock may not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of such securities by any person, for a period of 180 days immediately following commencement of sale of this offering subject to certain exceptions permitted by FINRA Rule 5110(e)(2).
| 71 |
| Table of Contents |
Bevilacqua PLLC has acted as our counsel in connection with the preparation of this prospectus. The validity of the shares of common stock covered by this prospectus will be passed upon by Lockett + Horwitz, A Professional Law Corporation. ArentFox Schiff LLP, Washington, DC, is acting as counsel to the underwriters.
The consolidated financial statements of Know Labs, Incorporated and subsidiaries as of September 30, 2021 and 2020 and for the two years then ended, have been included herein in reliance upon the report of BMP LLP, an independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement, of which this prospectus is a part, on Form S-1 with the SEC relating to this offering. This prospectus does not contain all of the information in the registration statement and the exhibits included with the registration statement. For further information pertaining to us and the common stock to be sold in this offering, you should refer to the registration statement and its exhibits. References in this prospectus to any of our contracts, agreements or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contracts, agreements or documents. You can read our SEC filings, including the registration statement, on the internet at the SEC’s website. The address of that site is http://www.sec.gov.
We are subject to the informational requirements of the Exchange Act, and, in accordance with the Exchange Act, we file reports, proxy and information statements and other information with the SEC. Such annual, quarterly and special reports, proxy and information statements and other information can be inspected and copied at the locations set forth above. We also make these documents publicly available, free of charge, on our website at www.knowlabs.co. as soon as reasonably practicable after filing such documents with the SEC. Information on, or accessible through, our website is not part of this prospectus.
| 72 |
| Table of Contents |
FINANCIAL STATEMENTS
|
|
| Page |
|
|
|
|
|
|
| Unaudited Consolidated Financial Statements for the Three and Nine Months Ended June 30, 2022 and 2021 |
| F-2 |
|
| Consolidated Balance Sheets as of June 30, 2022 (unaudited) and September 30, 2021 |
| F-2 |
|
| Consolidated Statements of Operations for the Three and Nine Months Ended June 30, 2022 and 2021 |
| F-3 |
|
|
| F-4 |
|
|
| Consolidated Statements of Cash Flows for the Nine Months Ended June 30, 2022 and 2021 |
| F-5 |
|
|
| F-6 |
|
|
|
|
|
|
|
| Audited Consolidated Financial Statements for the Years Ended September 30, 2021 and 2020 |
|
|
|
|
| F-20 |
|
|
| Consolidated Balance Sheets as of September 30, 2021 and 2020 |
| F-22 |
|
| Consolidated Statements of Operations for the Years Ended September 30, 2021 and 2020 |
| F-23 |
|
|
| F-24 |
|
|
| Consolidated Statements of Cash Flows for the Years Ended September 30, 2021 and 2020 |
| F-25 |
|
|
| F-26 |
|
| F-1 |
| Table of Contents |
KNOW LABS, INC.
UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
THREE AND NINE MONTHS ENDED JUNE 30, 2022 AND 2021
KNOW LABS, INCORPORATED AND SUBSIDIARIES
Unaudited
|
|
| June 30, 2022 |
|
| September 30, 2021 (1) |
|
||
| ASSETS |
| Unaudited |
|
|
|
|||
|
|
|
|
|
|
|
|
||
| CURRENT ASSETS: |
|
|
|
|
|
|
||
| Cash and cash equivalents |
| $ | 8,351,945 |
|
| $ | 12,258,218 |
|
| Accounts receivable- related party |
|
| 46,146 |
|
|
| - |
|
| Total current assets |
|
| 8,398,091 |
|
|
| 12,258,218 |
|
|
|
|
|
|
|
|
|
|
|
| PROPERTY AND EQUIPMENT, NET |
|
| 953,378 |
|
|
| 328,504 |
|
|
|
|
|
|
|
|
|
|
|
| OTHER ASSETS |
|
|
|
|
|
|
|
|
| Other assets |
|
| 13,767 |
|
|
| 13,767 |
|
| Operating lease right of use asset |
|
| 262,242 |
|
|
| 289,002 |
|
|
|
|
|
|
|
|
|
|
|
| TOTAL ASSETS |
| $ | 9,627,478 |
|
| $ | 12,889,491 |
|
|
|
|
|
|
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CURRENT LIABILITIES: |
|
|
|
|
|
|
|
|
| Accounts payable - trade |
| $ | 405,423 |
|
| $ | 419,093 |
|
| Accrued expenses |
|
| 533,993 |
|
|
| 893,137 |
|
| Accrued expenses - related parties |
|
| 1,270,652 |
|
|
| 421,599 |
|
| Convertible notes payable, net |
|
| 2,255,066 |
|
|
| 9,191,155 |
|
| Current portion of operating lease right of use liability |
|
| 145,815 |
|
|
| 112,371 |
|
| Total current liabilities |
|
| 4,610,949 |
|
|
| 11,037,355 |
|
|
|
|
|
|
|
|
|
|
|
| NON-CURRENT LIABILITIES: |
|
|
|
|
|
|
|
|
| Notes payable- PPP loans |
|
| - |
|
|
| 431,803 |
|
| Operating lease right of use liability, net of current portion |
|
| 97,261 |
|
|
| 178,170 |
|
| Total non-current liabilities |
|
| 97,261 |
|
|
| 609,973 |
|
|
|
|
|
|
|
|
|
|
|
| COMMITMENTS AND CONTINGENCIES (Note 11) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
| Preferred stock - $0.001 par value, 5,000,000 shares authorized, Series C and D shares issued and outstanding as follows: |
|
|
|
|
|
|
|
|
| Series C Convertible Preferred stock $0.001 par value, 1,785,715 shares authorized, 1,785,715 shares issued and outstanding at 6/30/2022 and 9/30/2021, respectively |
|
| 1,790 |
|
|
| 1,790 |
|
| Series D Convertible Preferred stock $0.001 par value, 1,016,014 shares authorized, 1,016,004 shares issued and outstanding at 6/30/2022 and 9/30/2021, respectively |
|
| 1,015 |
|
|
| 1,015 |
|
| Common stock - $0.001 par value, 200,000,000 shares authorized, 43,802,147 and 35,166,551 shares issued and outstanding at 6/30/2022 and 9/30/2021, respectively |
|
| 43,803 |
|
|
| 35,168 |
|
| Additional paid in capital |
|
| 100,699,797 |
|
|
| 82,530,684 |
|
| Accumulated deficit |
|
| (95,827,137 | ) |
|
| (81,326,494 | ) |
| Total stockholders' equity |
|
| 4,919,268 |
|
|
| 1,242,163 |
|
|
|
|
|
|
|
|
|
|
|
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
| $ | 9,627,478 |
|
| $ | 12,889,491 |
|
(1) Derived from the audited consolidated balance sheet.
The accompanying notes are an integral part of these consolidated financial statements.
| F-2 |
| Table of Contents |
NOW LABS, INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
Unaudited
|
|
| Three Months Ended, |
|
| Nine Months Ended, |
|
||||||||||
|
|
| June 30, 2022 |
|
| June 30, 2021 |
|
| June 30, 2022 |
|
| June 30, 2021 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| REVENUE- DIGITAL ASSET SALES |
| $ | - |
|
| $ | - |
|
| $ | 4,360,087 |
|
| $ | - |
|
| OPERATING EXPENSES- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| RESEARCH AND DEVELOPMENT EXPENSES |
|
| 1,272,537 |
|
|
| 868,584 |
|
|
| 3,406,996 |
|
|
| 3,094,123 |
|
| SELLING, GENERAL AND ADMINISTRATIVE EXPENSES |
|
| 1,588,823 |
|
|
| 994,221 |
|
|
| 4,253,997 |
|
|
| 4,934,085 |
|
| SELLING AND TRANSACTIONAL COSTS FOR DIGITAL ASSETS |
|
| 164,093 |
|
|
| - |
|
|
| 3,436,955 |
|
|
| - |
|
| Total operating expenses |
|
| 3,025,453 |
|
|
| 1,862,805 |
|
|
| 11,097,948 |
|
|
| 8,028,208 |
|
| OPERATING LOSS |
|
| (3,025,453 | ) |
|
| (1,862,805 | ) |
|
| (6,737,861 | ) |
|
| (8,028,208 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| OTHER INCOME (EXPENSE): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
|
| (239,760 | ) |
|
| (5,228,058 | ) |
|
| (8,024,709 | ) |
|
| (9,735,604 | ) |
| Other income |
|
| 261,927 |
|
|
| - |
|
|
| 261,927 |
|
|
| - |
|
| Total other (expense), net |
|
| 22,167 |
|
|
| (5,228,058 | ) |
|
| (7,762,782 | ) |
|
| (9,735,604 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| LOSS BEFORE INCOME TAXES |
|
| (3,003,286 | ) |
|
| (7,090,863 | ) |
|
| (14,500,643 | ) |
|
| (17,763,812 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income tax expense |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NET LOSS |
| $ | (3,003,286 | ) |
| $ | (7,090,863 | ) |
| $ | (14,500,643 | ) |
| $ | (17,763,812 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic and diluted loss per share |
| $ | (0.07 | ) |
| $ | (0.23 | ) |
| $ | (0.37 | ) |
| $ | (0.64 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average shares of common stock outstanding- basic and diluted |
|
| 43,760,904 |
|
|
| 31,052,229 |
|
|
| 39,032,860 |
|
|
| 27,651,679 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
| Table of Contents |
KNOW LABS, INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' (DEFICIT) EQUITY
Unaudited
|
|
| Series C Convertible |
|
| Series D Convertible |
|
|
|
|
|
|
|
| Additional |
|
|
|
|
| Total Stockholders |
|
|||||||||||||||
|
|
| Preferred Stock |
|
| Preferred Stock |
|
| Common Stock |
|
| Paid in |
|
| Accumulated |
|
| 'Equity |
|
||||||||||||||||||
|
|
| Shares |
|
| $ |
|
| Shares |
|
| $ |
|
| Shares |
|
| $ |
|
| Capital |
|
| Deficit |
|
| (Deficit) |
|
|||||||||
| Balance as of October 1, 2020 |
|
| 1,785,715 |
|
| $ | 1,790 |
|
|
| 1,016,004 |
|
| $ | 1,015 |
|
|
| 24,804,874 |
|
| $ | 24,807 |
|
| $ | 54,023,758 |
|
| $ | (55,966,281 | ) |
| $ | (1,914,911 | ) |
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 175,442 |
|
|
| - |
|
|
| 175,442 |
|
| Conversion of debt offering and accrued interest (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 561,600 |
|
|
| 562 |
|
|
| 561,038 |
|
|
| - |
|
|
| 561,600 |
|
| Issuance of warrant for services to related party |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,811,691 |
|
|
| - |
|
|
| 1,811,691 |
|
| Issuance of common stock for exercise of warrants |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 3,750 |
|
|
| 4 |
|
|
| 4,684 |
|
|
| - |
|
|
| 4,688 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (5,299,331 | ) |
|
| (5,299,331 | ) |
| Balance as of December 31, 2020 |
|
| 1,785,715 |
|
|
| 1,790 |
|
|
| 1,016,004 |
|
|
| 1,015 |
|
|
| 25,370,224 |
|
|
| 25,372 |
|
|
| 56,576,613 |
|
|
| (61,265,612 | ) |
|
| (4,660,822 | ) |
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 127,407 |
|
|
| - |
|
|
| 127,407 |
|
| Conversion of debt offering and accrued interest (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 210,600 |
|
|
| 211 |
|
|
| 210,395 |
|
|
| - |
|
|
| 210,606 |
|
| Beneficial conversion feature (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 9,769,683 |
|
|
| - |
|
|
| 9,769,683 |
|
| Issuance of warrants to debt holders (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 4,439,317 |
|
|
| - |
|
|
| 4,439,317 |
|
| Issuance of warrants for services related to debt offering (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,667,281 |
|
|
| - |
|
|
| 1,667,281 |
|
| Issuance of common stock for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 97,000 |
|
|
| 97 |
|
|
| 202,723 |
|
|
| - |
|
|
| 202,820 |
|
| Issuance of warrant for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 382,566 |
|
|
| - |
|
|
| 382,566 |
|
| Issuance of common stock for exercise of warrants |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 2,579,643 |
|
|
| 2,578 |
|
|
| 645,938 |
|
|
| - |
|
|
| 648,516 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (5,373,618 | ) |
|
| (5,373,618 | ) |
| Balance as of March 31, 2021 |
|
| 1,785,715 |
|
|
| 1,790 |
|
|
| 1,016,004 |
|
|
| 1,015 |
|
|
| 28,257,467 |
|
|
| 28,258 |
|
|
| 74,021,923 |
|
|
| (66,639,230 | ) |
|
| 7,413,756 |
|
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 267,665 |
|
|
| - |
|
|
| 267,665 |
|
| Conversion of debt offering and accrued interest (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 5,318,460 |
|
|
| 5,318 |
|
|
| 5,320,535 |
|
|
| - |
|
|
| 5,325,853 |
|
| Issuance of common stock for exercise of warrants |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 126,867 |
|
|
| 127 |
|
|
| 59,873 |
|
|
| - |
|
|
| 60,000 |
|
| Issuance of common stock for stock option exercises |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 16,875 |
|
|
| 17 |
|
|
| 23,327 |
|
|
| - |
|
|
| 23,344 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (7,090,863 | ) |
|
| (7,090,863 | ) |
| Balance as of June 30, 2021 |
|
| 1,785,715 |
|
| $ | 1,790 |
|
|
| 1,016,004 |
|
| $ | 1,015 |
|
|
| 33,719,669 |
|
| $ | 33,720 |
|
| $ | 79,693,322 |
|
| $ | (73,730,093 | ) |
| $ | 5,999,754 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance as of October 1, 2021 |
|
| 1,785,715 |
|
|
| 1,790 |
|
|
| 1,016,004 |
|
|
| 1,015 |
|
|
| 35,166,551 |
|
|
| 35,168 |
|
|
| 82,530,684 |
|
|
| (81,326,494 | ) |
|
| 1,242,163 |
|
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 204,170 |
|
|
| - |
|
|
| 204,170 |
|
| Issuance of common stock for exercise of warrants |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 801,486 |
|
|
| 801 |
|
|
| 765,685 |
|
|
| - |
|
|
| 766,486 |
|
| Issuance of common stock for stock option exercises |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,875 |
|
|
| 2 |
|
|
| 2,342 |
|
|
| - |
|
|
| 2,344 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (5,356,619 | ) |
|
| (5,356,619 | ) |
| Balance as of December 31, 2021 |
|
| 1,785,715 |
|
|
| 1,790 |
|
|
| 1,016,004 |
|
|
| 1,015 |
|
|
| 35,969,912 |
|
|
| 35,971 |
|
|
| 83,502,881 |
|
|
| (86,683,113 | ) |
|
| (3,141,456 | ) |
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 432,481 |
|
|
| - |
|
|
| 432,481 |
|
| Conversion of debt offering and accrued interest (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 7,672,860 |
|
|
| 7,673 |
|
|
| 15,338,047 |
|
|
| - |
|
|
| 15,345,720 |
|
| Issuance of common stock for stock option exercises |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 5,000 |
|
|
| 5 |
|
|
| 8,995 |
|
|
| - |
|
|
| 9,000 |
|
| Issuance of common stock for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 90,000 |
|
|
| 90 |
|
|
| 152,910 |
|
|
| - |
|
|
| 153,000 |
|
| Issuance of warrant for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 71,220 |
|
|
| - |
|
|
| 71,220 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (6,140,738 | ) |
|
| (6,140,738 | ) |
| Balance as of March 31, 2022 |
|
| 1,785,715 |
|
|
| 1,790 |
|
|
| 1,016,004 |
|
|
| 1,015 |
|
|
| 43,737,772 |
|
|
| 43,739 |
|
|
| 99,506,534 |
|
|
| (92,823,851 | ) |
|
| 6,729,227 |
|
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 919,224 |
|
|
| - |
|
|
| 919,224 |
|
| Issuance of common stock for exercise of warrants |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 62,500 |
|
|
| 62 |
|
|
| 27,438 |
|
|
| - |
|
|
| 27,500 |
|
| Issuance of common stock for stock option exercises |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,875 |
|
|
| 2 |
|
|
| 2,341 |
|
|
| - |
|
|
| 2,343 |
|
| Modification of warrant-interest expense |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 244,260 |
|
|
| - |
|
|
| 244,260 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (3,003,286 | ) |
|
| (3,003,286 | ) |
| Balance as of June 30, 2022 |
|
| 1,785,715 |
|
| $ | 1,790 |
|
|
| 1,016,004 |
|
| $ | 1,015 |
|
|
| 43,802,147 |
|
| $ | 43,803 |
|
| $ | 100,699,797 |
|
| $ | (95,827,137 | ) |
| $ | 4,919,268 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
| Table of Contents |
KNOW LABS, INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
Unaudited
|
|
| Nine Months Ended, |
|
|||||
|
|
| June 30, 2022 |
|
| June 30, 2021 |
|
||
|
|
|
|
|
|
|
|
||
| CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
||
| Net loss |
| $ | (14,500,643 | ) |
| $ | (17,763,812 | ) |
| Adjustments to reconcile net loss to net cash (used in) |
|
|
|
|
|
|
|
|
| operating activities |
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
| 218,683 |
|
|
| 166,094 |
|
| Issuance of common stock for services |
|
| 153,000 |
|
|
| 202,820 |
|
| Issuance of common stock warrants for services |
|
| 71,220 |
|
|
| - |
|
| Stock based compensation- warrants |
|
| - |
|
|
| 2,194,257 |
|
| Modification of warrant-interest expense |
|
| 244,260 |
|
|
| - |
|
| Stock based compensation- stock option grants |
|
| 1,555,875 |
|
|
| 570,514 |
|
| Right of use, net |
|
| (20,705 | ) |
|
| (1,853 | ) |
| Gain on forgiveness of notes payable - PPP loans |
|
| (252,700 | ) |
|
| - |
|
| Amortization of debt discount to interest expense |
|
| 7,272,911 |
|
|
| 9,071,592 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Accounts receivable- related party |
|
| (46,146 | ) |
|
| - |
|
| Other long-term assets |
|
| - |
|
|
| 11,413 |
|
| Accounts payable - trade and accrued expenses |
|
| 1,612,959 |
|
|
| 404,469 |
|
| NET CASH (USED IN) OPERATING ACTIVITIES |
|
| (3,691,286 | ) |
|
| (5,144,506 | ) |
|
|
|
|
|
|
|
|
|
|
| CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
| Purchase of research and development equipment |
|
| (843,557 | ) |
|
| (47,553 | ) |
| NET CASH (USED IN) INVESTING ACTIVITIES: |
|
| (843,557 | ) |
|
| (47,553 | ) |
|
|
|
|
|
|
|
|
|
|
| CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
| Proceeds from convertible notes payable |
|
| - |
|
|
| 14,209,000 |
|
| Payments for issuance costs from notes payable |
|
| - |
|
|
| (727,117 | ) |
| Proceeds from Simple Agreements for Future Equity |
|
| - |
|
|
| 340,000 |
|
| Proceeds from note payable - PPP |
|
| - |
|
|
| 205,633 |
|
| Settlement of notes payable- PPP loans |
|
| (179,103 | ) |
|
| - |
|
| Proceeds from issuance of common stock for stock options exercise |
|
| 13,687 |
|
|
| 23,344 |
|
| Proceeds from issuance of common stock for warrant exercise |
|
| 793,986 |
|
|
| 713,203 |
|
| NET CASH PROVIDED BY FINANCING ACTIVITIES |
|
| 628,570 |
|
|
| 14,764,063 |
|
|
|
|
|
|
|
|
|
|
|
| NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS |
|
| (3,906,273 | ) |
|
| 9,572,004 |
|
|
|
|
|
|
|
|
|
|
|
| CASH AND CASH EQUIVALENTS, beginning of period |
|
| 12,258,218 |
|
|
| 4,298,179 |
|
|
|
|
|
|
|
|
|
|
|
| CASH AND CASH EQUIVALENTS, end of period |
| $ | 8,351,945 |
|
| $ | 13,870,183 |
|
|
|
|
|
|
|
|
|
|
|
| Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
| Interest paid |
| $ | - |
|
| $ | - |
|
| Taxes paid |
| $ | - |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
|
| Non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
| $ | - |
|
| $ | 9,769,683 |
|
| Issuance of warrants to debt holders |
| $ | - |
|
| $ | 4,439,317 |
|
| Issuance of warrants for services related to debt offering |
| $ | - |
|
| $ | 1,667,281 |
|
| Cashless warrant exercise (fair value) |
| $ | - |
|
| $ | 493,601 |
|
| Conversion of debt |
| $ | 14,209,000 |
|
| $ | 5,638,275 |
|
| Conversion of accrued interest |
| $ | 1,136,720 |
|
| $ | 460,185 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
| Table of Contents |
KNOW LABS, INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The accompanying unaudited consolidated condensed financial statements have been prepared by Know Labs, Inc, (“the Company,” “us,” “we,” or “our”) in accordance with U.S. generally accepted accounting principles (“GAAP”) for interim financial reporting and rules and regulations of the Securities and Exchange Commission. Accordingly, certain information and footnote disclosures normally included in financial statements prepared in accordance with GAAP have been condensed or omitted. In the opinion of our management, all adjustments, consisting of only normal recurring accruals, necessary for a fair presentation of the financial position, results of operations, and cash flows for the fiscal periods presented have been included.
These financial statements should be read in conjunction with the audited financial statements and related notes included in our Annual Report filed on Form 10-K for the year ended September 30, 2021, filed with the Securities and Exchange Commission (“SEC”) on December 21, 2021. The results of operations for the nine months ended June 30, 2022 are not necessarily indicative of the results expected for the full fiscal year, or for any other fiscal period.
1. ORGANIZATION
Know Labs, Inc. was incorporated under the laws of the State of Nevada in 1998. The Company currently has authorized 205,000,000 shares of capital stock, of which 200,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are shares preferred stock, par value $0.001 per share. At the annual shareholder meeting held on October 15, 2021, our authorized shares of common stock was increased to 200,000,000 shares of voting common stock, par value $0.001 per share.
Know Labs is focused on the development and commercialization of proprietary biosensor technologies which, when paired with our artificial intelligence, or AI, deep learning platform, are capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify and measure the unique “signature” of said materials or analytes. The Company call these our “Bio-RFID™” technology platform when pertaining to radio and microwave spectroscopy; and “ChromaID” technology platform when pertaining to optical spectroscopy. The data obtained with our biosensor technology is analyzed with our trade secret algorithms which are driven by our AI deep learning platform. There are a significant number of analytes in the human body that relate to health and wellness. The Company’s focus is upon those analytes relating to human health, the identification of which provide diagnostic information and require, by their nature, clearance by the United States Food and Drug Administration.
On April 30, 2020, the Company approved and ratified the incorporation of Particle, Inc. Particle is focused on the development and commercialization of our extensive intellectual property relating to electromagnetic energy outside of the medical diagnostic arena which remains the parent company’s singular focus. Since incorporation, Particle has engaged in research and development activities on threaded light bulbs that have a warm white light and can inactivate germs, including bacteria and viruses. It is now looking for partners to take the product to market.
On September 17, 2021, the Company incorporated AI Mind, Inc. AI Mind is focused on monetizing the AI Deep Learning Platform. Since incorporation it initially focused on creating graphical images which were sold as Non Fungible Tokens (“NFTs”). During the nine months ended June 30, 2022, the Company’s artificial intelligence (AI) Deep Learning Platform began generating revenue from digital asset sales of NFT’s and had sales of $4,360,087.
2. LIQUIDITY AND GOING CONCERN
The Company has cash and cash equivalents of $8,351,945 and net working capital of $6,042,208 (exclusive of convertible notes payable) as of June 30, 2022. The Company anticipates that it will record losses from operations for the foreseeable future. The Company believes that it has enough available cash to operate until June 30, 2023. As of June 30, 2022, the Company’s accumulated deficit was $95,827,137. The Company has had limited capital resources and intends to seek additional cash via equity and debt offerings.
On July 29, 2022, the Company filed a Form S-1 registration statement for a proposed new offering of 3 million shares of its common stock with an anticipated offering price of $2.00 per share. Concurrent with, and as a condition to, the offering, Know Labs will apply to uplist its shares to the NYSE American Exchange. The closing of this offering is contingent upon the successful listing of our common stock on NYSE American.
The proceeds of warrants currently outstanding, which could be exercised on a cash basis, may generate potential proceeds of up to $16,383,908.
If the new offering referenced above is not successful or the warrants are not exercised, the cash conditions may raise substantial doubt about our ability to continue as a going concern. The audit report prepared by the Company’s independent registered public accounting firm relating to our consolidated financial statements for the year ended September 30, 2021 did not include an explanatory paragraph expressing the substantial doubt about the Company’s ability to continue as a going concern.
| F-6 |
| Table of Contents |
3. SIGNIFICANT ACCOUNTING POLICIES: ADOPTION OF ACCOUNTING STANDARDS
Basis of Presentation – The accompanying consolidated financial statements include the accounts of the Company. Intercompany accounts and transactions have been eliminated. The preparation of these unaudited condensed consolidated financial statements were prepared in conformity with U.S. generally accepted accounting principles (“GAAP”).
Principles of Consolidation – The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, Particle, Inc. and AI Mind, Inc. Inter-Company items and transactions have been eliminated in consolidation.
Cash and Cash Equivalents
– The Company classifies highly liquid temporary investments with an original maturity of three months or less when purchased as cash equivalents. The Company maintains cash balances at various financial institutions. Balances at US banks are insured by the Federal Deposit Insurance Corporation up to $250,000. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risk for cash on deposit.
Equipment – Equipment consists of machinery, leasehold improvements, furniture and fixtures and software, which are stated at cost less accumulated depreciation and amortization. Depreciation is computed by the straight-line method over the estimated useful lives or lease period of the relevant asset, generally 2-5 years, except for leasehold improvements which are depreciated over 5 years.
Long-Lived Assets – The Company reviews its long-lived assets for impairment annually or when changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Long-lived assets under certain circumstances are reported at the lower of carrying amount or fair value. Assets to be disposed of and assets not expected to provide any future service potential to the Company are recorded at the lower of carrying amount or fair value (less the projected cost associated with selling the asset). To the extent carrying values exceed fair values, an impairment loss is recognized in operating results.
Intangible Assets – Intangible assets are capitalized and amortized on a straight-line basis over their estimated useful life, if the life is determinable. If the life is not determinable, amortization is not recorded. We regularly perform reviews to determine if facts and circumstances exist which indicate that the useful lives of our intangible assets are shorter than originally estimated or the carrying amount of these assets may not be recoverable. When an indication exists that the carrying amount of intangible assets may not be recoverable, we assess the recoverability of our assets by comparing the projected undiscounted net cash flows associated with the related asset or group of assets over their remaining lives against their respective carrying amounts. Such impairment test is based on the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. Impairment, if any, is based on the excess of the carrying amount over the estimated fair value of those assets.
Revenue Recognition - The Company determines revenue recognition from contracts with customers through the following steps:
|
| · | identification of the contract, or contracts, with the customer; |
|
|
|
|
|
| · | identification of the performance obligations in the contract; |
|
|
|
|
|
| · | determination of the transaction price; |
|
|
|
|
|
| · | allocation of the transaction price to the performance obligations in the contract; and |
|
|
|
|
|
| · | recognition of the revenue when, or as, the Company satisfies a performance obligation. |
Revenue is recognized when control of the promised goods or services is transferred to the customers, in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods or services. During the nine months ended June 30, 2022, the Company’s artificial intelligence (AI) Deep Learning Platform began generating revenue from digital asset sales of NFT’s. The Company engineering team, using its research date, AI and proprietary algorithms, produced NFT’s in the form of digital art. The NFT’s produced had no recorded cost basis.
Digital Asset Sales - Revenue includes sale of NFT’s in the form of digital art generated from the Company’s artificial intelligence Deep Learning Platform. The Company uses the NFT exchange, OpenSea, to facilitate the transaction with the customer. The Company, through OpenSea, has custody and control of the NFT prior to the delivery to the customer and records revenue at the point in time when the NFT is delivered to the customer and the customer pays. The Company has no obligations for returns, refunds or warranty after the NFT sale. The customer pays in the form of transferring the crypto currency digital asset, Ethereum. The value of the sale is determined based on the value of the Ethereum crypto currency received as consideration. Payment is required before the NFT is delivered. Each NFT that is generated produces a unique identifying code. The Company also earns a royalty of up to 10%, when an NFT is resold by its owner in a secondary market transaction. The Company recognizes this royalty as revenue when the transaction is consummated, and they receive compensation.
After the sale of the NFT, the Ethereum is converted to US dollars as soon as practically possible. The Company records the total value of the gross NFT sale in revenue. Costs incurred in connection with the NFT transaction are recorded in the statement of operations as Selling and Transactional Cost of Digital Assets and include costs to outside consultants, estimated employee and CEO special bonus compensation, digital asset conversion losses and estimated sales and use tax. The amount totaled $164,093 and $3,436,955 for the three and nine months ended June 30, 2022, respectively.
| F-7 |
| Table of Contents |
Research and Development Expenses – Research and development expenses consist of the cost of employees, consultants and contractors who design, engineer and develop new products and processes as well as materials, supplies and facilities used in producing prototypes.
The Company’s current research and development efforts are primarily focused on improving our Bio-RFID technology, extending its capacity and developing new and unique applications for this technology. As part of this effort, the Company conducts on-going laboratory testing to ensure that application methods are compatible with the end-user and regulatory requirements, and that they can be implemented in a cost-effective manner. The Company also is actively involved in identifying new applications. The Company’s current internal team along with outside consultants has considerable experience working with the application of the Company’s technologies and their applications. The Company engages third party experts as required to supplement our internal team. The Company believes that continued development of new and enhanced technologies is essential to our future success. The Company incurred expenses of $3,406,996, $3,969,972 and $2,033,726 for the nine months ended June 30, 2022 and the years ended September 30, 2021 and 2020, respectively, on development activities.
Advertising
– Advertising costs are charged to selling, general and administrative expenses as incurred. Advertising and marketing costs for the nine months ended June 30, 2022 and 2021 were $514,401 and $240,000, respectively.
Fair Value Measurements and Financial Instruments – ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
| Level 1 – Quoted prices in active markets for identical assets and liabilities; |
| Level 2 – Inputs other than level one inputs that are either directly or indirectly observable; and.
Level 3 - Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The recorded value of other financial assets and liabilities, which consist primarily of cash and cash equivalents, accounts receivable, other current assets, and accounts payable and accrued expenses approximate the fair value of the respective assets and liabilities as of June 30, 2022 and September 30, 2021 are based upon the short-term nature of the assets and liabilities.
The Company has a money market account which is considered a level 1 asset. The balance as of June 30, 2022 and September 30, 2021 was $6,041,016 and $12,217,714, respectively.
Derivative Financial Instruments –Pursuant to ASC 815 “Derivatives and Hedging”, the Company evaluates all of its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. The Company then determines if embedded derivative must be bifurcated and separately accounted for. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. For stock-based derivative financial instruments, the Company uses a Black-Scholes-Merton option pricing model to value the derivative instruments at inception and on subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within twelve months of the balance sheet date.
The Company determined that the conversion features for purposes of bifurcation within convertible notes payable were immaterial and there was no derivative liability to be recorded as of June 30, 2022 and September 30, 2021.
Stock Based Compensation - The Company has share-based compensation plans under which employees, consultants, suppliers and directors may be granted restricted stock, as well as options and warrants to purchase shares of Company common stock at the fair market value at the time of grant. Stock-based compensation cost to employees is measured by the Company at the grant date, based on the fair value of the award, over the requisite service period under ASC 718. For options issued to employees, the Company recognizes stock compensation costs utilizing the fair value methodology over the related period of benefit.
Convertible Securities – Based upon ASC 815-15, the Company has adopted a sequencing approach regarding the application of ASC 815-40 to convertible securities. The Company will evaluate our contracts based upon the earliest issuance date. In the event partial reclassification of contracts subject to ASC 815-40-25 is necessary, due to the Company’s inability to demonstrate we have sufficient shares authorized and unissued, shares will be allocated on the basis of issuance date, with the earliest issuance date receiving first allocation of shares. If a reclassification of an instrument were required, it would result in the instrument issued latest being reclassified first.
| F-8 |
| Table of Contents |
Net Loss per Share
– Under the provisions of ASC 260, “Earnings Per Share,” basic loss per common share is computed by dividing net loss available to common stockholders by the weighted average number of shares of common stock outstanding for the periods presented. Diluted net loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock. As of June 30, 2022, the Company had 43,802,147 shares of common stock issued and outstanding. As of June 30, 2022, there were options outstanding for the purchase of 20,927,370 common shares (including unearned stock option grants totaling 11,550,745 shares related to performance targets), warrants for the purchase of 21,651,513 common shares, and 8,108,356 shares of our common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock. In addition, the Company currently has 9,020,264 common shares at the current price of $0.25 per share reserved and are issuable upon conversion of convertible debentures of $2,255,066. All of which could potentially dilute future earnings per share but are excluded from the June 30, 2022, calculation of net loss per share because their impact is antidilutive.
As of June 30, 2021, the Company had 33,719,669 shares of common stock issued and outstanding. As of June 30, 2021, there were options outstanding for the purchase of 14,650,120 common shares (including unearned stock option grants totaling 11,775,745 shares related to performance targets), warrants for the purchase of 23,169,587 common shares, and 8,108,356 shares of the Company’s common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock. In addition, the Company currently has 16,124,764 common shares (9,020,264 common shares at the current price of $0.25 per share and 7,104,500 common shares at the current price of $2.00 per share) reserved and are issuable upon conversion of convertible debentures of $16,464,066. All of which could potentially dilute future earnings per share but are excluded from the June 30, 2021 calculation of net loss per share because their impact is antidilutive.
Comprehensive loss – Comprehensive loss is defined as the change in equity of a business during a period from non-owner sources. There were no differences between net loss for the nine months ended June 30, 2022 and 2021 and comprehensive loss for those periods.
Dividend Policy – The Company has never paid any cash dividends and intends, for the foreseeable future, to retain any future earnings for the development of our business. Our future dividend policy will be determined by the board of directors on the basis of various factors, including our results of operations, financial condition, capital requirements and investment opportunities.
Use of Estimates – The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Recent Accounting Pronouncements In August 2020, the FASB issued Accounting Standards Update (“ASU”) No. 2020-06, Debt -- debt with Conversion and Other Options (Subtopic470-20) and Derivatives and Hedging --Contracts in Entity’ Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’ Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for the derivative scope exception, and it simplifies the diluted earnings per share calculation in certain areas. The new standard is effective for the Company on October 1, 2021. Adoption of the ASU did not have an impact to the Company’s financial position, results of operations or cashflows.
Based on the Company’s review of accounting standard updates issued since the filing of the June 30, 2022 Form 10-Q, there have been no other newly issued or newly applicable accounting pronouncements that have had, or are expected to have, a significant impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
4. Artificial Intelligence (AI) Deep Learning Platform
AI Revenue
During the nine months ended June 30, 2022, the Company’s artificial intelligence (AI) Deep Learning Platform began generating revenue from digital asset sales of NFT’s and had sales of $4,360,087. The Company’s sales of NFT’s are generated using the NFT digital exchange, OpenSea. Customers purchasing the NFT’s must make payments in the crypto currency, Ethereum. The Ethereum is received into a digital wallet and then moved to an account at Coinbase where the Ethereum is converted to U.S. dollars. During the three months ended December 31, 2021, the Company was not able to establish a digital wallet and corporate account at Coinbase in order to receive the Ethereum. The Company used the digital wallet and Coinbase account of the Company’s CEO. The Company and the CEO executed an assignment of his account to the Company while the Company establishes its own Coinbase account. All proceeds received from the sale of NFT were deposited in the CEO’s personal digital accounts. As of December 31, 2021 the Company had recorded an accounts receivable-related party of $3,124,581 for the cash it expected to receive from the CEO’s personal digital account.
| F-9 |
| Table of Contents |
The Company has established a digital wallet and corporate account at Circle in order to receive the Ethereum and then convert it to cash. During 2022, the Company received $2,908,551 of Ethereum which was converted to cash and recorded a reduction in value of $169,884 related to the decline in value of the Ethereum. The accounts receivable-related party was $46,146 as of June 30, 2022. As of June 30, 2022, accrued expenses include $436,378 of expenses, primarily sales and use tax and other expenses. As of June 30, 2022 accrued expenses-related party include $799,353 of unpaid compensation that was due and payable to the CEO for the NFT revenue. This unpaid compensation was paid in July 2022. During 2021, approximately $1.3 million of the selling and transactional costs for the digital assets was paid through the CEO’s personal digital asset account including approximately $1.075 million which was paid to a consultant via the transfer of Ethereum.
5. PROPERTY AND EQUIPMENT
Property and equipment as of June 30, 2022 and September 30, 2021 was comprised of the following:
|
|
| Estimated |
| June 30, |
|
| September 30, |
|
||
|
|
| Useful Lives |
| 2022 |
|
| 2021 |
|
||
| Machinery and equipment |
| 2-3 years |
| $ | 1,498,355 |
|
| $ | 654,798 |
|
| Leasehold improvements |
| 5 years |
|
| 3,612 |
|
|
| 3,612 |
|
| Furniture and fixtures |
| 5 years |
|
| 26,855 |
|
|
| 26,855 |
|
| Less: accumulated depreciation |
|
|
|
| (575,444 | ) |
|
| (356,761 | ) |
|
|
|
|
| $ | 953,378 |
|
| $ | 328,504 |
|
Total depreciation expense was $218,683 and $64,980 for the nine months ended June 20, 2022 and 2021, respectively. $207,440 and $10,918 of depreciation was recorded for research and development and selling, general and administrative purposes, respectively, for the nine months ended June 30, 2022. $0 and $64,980 of depreciation was recorded for research and development and selling, general and administrative purposes, respectively, for the nine months ended June 30, 2021.
6. LEASES
The Company has entered into operating leases for office and development facilities. These leases have terms which range from two to three years and include options to renew. These operating leases are listed as separate line items on the Company's June 30, 2022 and September 30, 2021 Consolidated Balance Sheets and represent the Company’s right to use the underlying asset for the lease term. The Company’s obligation to make lease payments are also listed as separate line items on the Company's June 30, 2022 and September 30, 2021 Consolidated Balance Sheets. Based on the present value of the lease payments for the remaining lease term of the Company's existing leases, the Company recognized right-of-use assets and lease liabilities for operating leases of approximately $262,242 as of June 30, 2022. Operating lease right-of-use assets and liabilities commencing after October 1, 2018 are recognized at commencement date based on the present value of lease payments over the lease term. During years ended June 30, 2022 and September 30, 2021, the Company amended two leases and recognized the rent payments as an expense in the current period. As of June 30, 2022 and September 30, 2021, total operating lease liabilities for remaining long term lease was approximately $243,076 and $290,000, respectively. In the nine months ended June 30, 2022 and 2021, the Company recognized approximately $130,767 and $102,741, respectively in total lease costs for the leases.
Because the rate implicit in each lease is not readily determinable, the Company uses its incremental borrowing rate to determine the present value of the lease payments.
Information related to the Company's operating right-of-use assets and related lease liabilities as of and for the year ended June 30, 2022 was as follows:
Cash paid for ROU operating lease liability $159,112
Weighted-average remaining lease term 20 months
Weighted-average discount rate 7%
| F-10 |
| Table of Contents |
The minimum future lease payments as of June 30, 2022 are as follows:
| Years Ended June 30, |
| $ |
|
|
| 2023 |
| $ | 156,253 |
|
| 2024 |
|
| 118,602 |
|
| Total remaining payments |
|
| 274,855 |
|
| Less Imputed Interest |
|
| (12,629 | ) |
| Total lease liability |
| $ | 262,226 |
|
7. CONVERTIBLE NOTES PAYABLE AND NOTE PAYABLE
Convertible notes payable as of June 30, 2022 and September 30, 2021 consisted of the following:
Convertible Promissory Notes with Clayton A. Struve
The Company owes Clayton A. Struve $1,071,000 under convertible promissory or OID notes. The Company recorded accrued interest of $84,671 and $79,062 as of June 30, 2022 and September 30, 2021, respectively. On May 3, 2022, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2022.
Convertible Redeemable Promissory Notes with Ronald P. Erickson and J3E2A2Z
On March 16, 2018, the Company entered into a Note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 currently owing under the J3E2A2Z Notes was converted to a Convertible Redeemable Promissory Note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z Account Payable was converted into a Convertible Redeemable Promissory Note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of common stock of the Company for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. The warrants were valued at $110,545. Because the note is immediately convertible, the warrants and beneficial conversion were expensed as interest. The Company recorded accrued interest of $269,383 and $216,246 as of June 30, 2022 and September 30, 2021, respectively. On April 4, 2022, the Company approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to September 30, 2022.
Convertible Debt Offering
Beginning in 2019, the Company entered into series of debt offerings with similar and consistent terms. The Company issued Subordinated Convertible Notes and Warrants in a private placement to accredited investors, pursuant to a series of substantially identical Securities Purchase Agreements, Common Stock Warrants, and related documents. The notes are convertible into one share of common stock for each dollar invested in a Convertible Note Payable and automatically convert to common stock after one year. The convertible notes contain terms and conditions which are deemed to be a Beneficial Conversion Feature (BCF). Warrants are issued to purchase common stock with exercise prices of $1.20 and $2.40 per share and the number of warrants are equal to 50% of the convertible note balance. The Company compensates the placement agent with a cash fee and warrants. Through June 30, 2022, the Company has raised approximately $24 million through these offerings, of which $14,209,000 and $5,639,500 were raised in the years ended September 30, 2021 and 2020, respectively.
During the year ended September 30, 2021, the Company issued 6,091,960 shares of common stock related to the automatic conversion of Convertible Notes and interest from a private placement to accredited investors in 2020. The Convertible Notes and interested were automatically converted to Common Stock at $1.00 per share on the one year anniversary starting on October 17, 2020.
The Convertible Notes issued during the year ended September 30, 2021 are initially convertible into 7,104,500 shares of Common Stock, subject to certain adjustments, and the Warrants are initially exercisable for 3,552,250 shares of Common Stoc. As of June 30, 2022 all convertible notes and accrued interest had been converted to common stock.
The fair value of the Warrants issued to debt holders during the year ended September 30, 2021 was $4,439,317 on the date of issuance and was amortized over the one-year term of the Convertible Notes. The fair value of the warrants was recorded as debt discount (with an offset to APIC) and was amortized over the one-year term of the Convertible Notes.
In connection with the debt offering during the year ended September 30, 2021, the placement agent for the Convertible Notes and the Warrants received a cash fee of $727,117 and warrants to purchase 492,090 shares of the Company’s common stock, all based on 2-8% of gross proceeds to the Company. The warrants issued for these services had a fair value of $1,667,281 at the date of issuance. The fair value of the warrants was recorded as debt discount (with an offset to APIC) and will be amortized over the one-year term of the Convertible Notes. The $727,117 cash fee was recorded as issuance costs and was amortized over the one-year term of the related Convertible Notes.
| F-11 |
| Table of Contents |
During the year ended September 30, 2021, the Company recorded a debt discount of $9,769,683 associated with a beneficial conversion feature on the debt, which was accreted to interest expense using the effective interest method over the one-year term of the Convertible Notes.
During the nine months ended June 30, 2022, amortization related to the debt offerings of $7,272,911 and $4,184,657 of the beneficial conversion feature, warrants issued to debt holders and placement agent was recognized as interest expense in the consolidated statements of operations.
Convertible notes payable as of June 30, 2022 and September 30, 2021 are summarized below:
|
|
| June 30, 2022 |
|
| September 30, 2021 |
|
||
| Convertible note- Clayton A. Struve |
| $ | 1,071,000 |
|
| $ | 1,071,000 |
|
| Convertible note- Ronald P. Erickson and affiliates |
|
| 1,184,066 |
|
|
| 1,184,066 |
|
| 2020 Convertible notes |
|
| - |
|
|
| 5,639,500 |
|
| 2021 Convertible notes |
|
| 14,209,000 |
|
|
| 14,209,000 |
|
| Boustead fee refund (originally booked as contra debt) |
|
| - |
|
|
| 50,000 |
|
| Less conversions of notes |
|
| (14,209,000 | ) |
|
| (5,639,500 | ) |
| Less debt discount - BCF |
|
| - |
|
|
| (4,308,337 | ) |
| Less debt discount - warrants |
|
| - |
|
|
| (1,957,590 | ) |
| Less debt discount - warrants issued for services |
|
| - |
|
|
| (1,056,984 | ) |
|
|
| $ | 2,255,066 |
|
| $ | 9,191,155 |
|
Note Payable-PPP Loans
On April 30, 2020, the Company received $226,170 under the Paycheck Protection Program of the U.S. Small Business Administration’s 7(a) Loan Program pursuant to the Coronavirus, Aid, Relief and Economic Security Act (CARES Act), Pub. Law 116-136, 134 Stat. 281 (2020). As of March 31, 2022 and September 30, 2021, the Company recorded interest expense of $4,350 and $3,222, respectively. On April 27, 2022, the Company was notified by the SBA that the Company is required to repay principal of $98,106 and interest of $1,997. The remaining loan and accrued interest balances were forgiven. .
On February 1, 2021, the Company received $205,633 under the Paycheck Protection Program of the U.S. Small Business Administration’s 7(a) Loan Program pursuant to the Coronavirus, Aid, Relief and Economic Security Act (CARES Act), Pub. Law 116-136, 134 Stat. 281 (2020). As of June 30, 2022 and September 30, 2021, the Company recorded interest expense of $2,721 and $1,268, respectively. On June 11, 2022, the Company was notified by the SBA that the Company is required to repay principal of $78,843 and interest of $1,057. The remaining loan and accrued interest balances were forgiven.
As a result of a portion of these loans being forgiven, the Company recognized a gain on loan forgiveness of approximately $253,000 which is included in other income.
EQUITY
Authorized Capital Stock
The Company was incorporated under the laws of the State of Nevada in 1998. The Company has authorized 205,000,000 shares of capital stock, of which 200,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are “blank check” preferred stock, par value $0.001 per share. As of June 30, 2022, the Company had 43,826,781 shares of common stock and 2,801,719 shares of preferred stock issued and outstanding.
As of June 30, 2022, there were options outstanding for the purchase of 20,927,370 common shares (including unearned stock option grants totaling 11,550,745 shares related to performance targets), warrants for the purchase of 21,651,513 common shares, and 8,108,356 shares of our common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock. In addition, the Company currently has 9,020,264 common shares at the current price of $0.25 per share reserved and are issuable upon conversion of convertible debentures of $2,255,066. All of which could potentially dilute future earnings per share but are excluded from the June 30, 2022, calculation of net loss per share because their impact is antidilutive.
Annual Shareholder Meeting
On October 15, 2021, the Company held its annual shareholder meeting. The Company’s shareholders approved and adopted various motions as detailed in the Company’s Form 8-K that was filed with the SEC on October 19, 2021.
| F-12 |
| Table of Contents |
Second Amended and Restated Bylaws
On October 15, 2021, the shareholders of the Company approving the Second Amended and Restated Bylaws effective October 15, 2021.
Certificate of Amendment to Articles of Incorporation
On December 6, 2021, the Company received approval from the State of Nevada for a Certificate of Amendment to the Articles of Incorporation related to the increase in the number of authorized common shares.
Series C and D Preferred Stock and Warrants
On August 5, 2016, the Company closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve, an accredited investor for the purchase of $1,250,000 of preferred stock with a conversion price of $0.70 per share. The preferred stock has a yield of 8% and an ownership blocker of 4.99%. In addition, Mr. Struve received a five-year warrant to acquire 1,785,714 shares of common stock at $0.70 per share. On August 14, 2017, the price of the Series C Stock were adjusted to $0.25 per share pursuant to the documents governing such instruments. On June 30, 2022 and September 30, 2021 there are 1,785,715 Series C Preferred shares outstanding. On May 3, 2022, the Company approved the Extension of Warrant Agreement with Clayton Struve, extending the exercise dates to August 4, 2024.
As of June 30, 2022 and September 30, 2021, the Company has $750,000 of Series D Preferred Stock outstanding with Clayton A. Struve, an accredited investor. On August 14, 2017, the price of the Series D Stock were adjusted to $0.25 per share pursuant to the documents governing such instruments. The Series D Preferred Stock is convertible into shares of common stock at a price of $0.25 per share or by multiplying the number of Series D Preferred Stock shares by the stated value and dividing by the conversion price then in effect, subject to certain diluted events, and has the right to vote the number of shares of common stock the Series D Preferred Stock would be issuable on conversion, subject to a 4.99% blocker. The Preferred Series D has an annual yield of 8% The Series D Preferred Stock is convertible into shares of common stock at a price of $0.25 per share or by multiplying the number of Series D Preferred Stock shares by the stated value and dividing by the conversion price then in effect, subject to certain diluted events, and has the right to vote the number of shares of common stock the Series D Preferred Stock would be issuable on conversion, subject to a 4.99% blocker. The Preferred Series D has an annual yield of 8% if and when dividends are declared.
Series F Preferred Stock
On August 1, 2018, the Company filed with the State of Nevada a Certificate of Designation establishing the Designations, Preferences, Limitations and Relative Rights of Series F Preferred Stock. The Designation authorized 500 shares of Series F Preferred Stock. The Series F Preferred Stock shall only be issued to the current Board of Directors on the date of the Designation’s filing and is not convertible into common stock. As set forth in the Designation, the Series F Preferred Stock has no rights to dividends or liquidation preference and carries rights to vote 100,000 shares of common stock per share of Series F upon a Trigger Event, as defined in the Designation. A Trigger Event includes certain unsolicited bids, tender offers, proxy contests, and significant share purchases, all as described in the Designation. Unless and until a Trigger Event, the Series F shall have no right to vote. The Series F Preferred Stock shall remain issued and outstanding until the date which is 731 days after the issuance of Series F Preferred Stock (“Explosion Date”), unless a Trigger Event occurs, in which case the Explosion Date shall be extended by 183 days. As of June 30, 2022 and September 30, 2021, there are no Series F shares outstanding.
Securities Subject to Price Adjustments
If in the future, if the Company sells its common stock at a price below $0.25 per share, the conversion price of 8,108,356 outstanding shares of Series C and D Preferred Stock would adjust below $0.25 per share pursuant to the documents governing such instruments. In addition, the conversion price of Convertible Notes Payable in the principal amount of $2,255,066, that convert into 9,020,264 shares of our common stock at $0.25 per share and the exercise price of certain outstanding warrants to purchase 10,284,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments. Warrants totaling 4,487,207 would adjust below $1.20 per share and warrants totaling 3,954,625 would adjust below $2.40 per share, in each case pursuant to the documents governing such instruments.
Common Stock
All of the offerings and sales described below were deemed to be exempt under Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act. No advertising or general solicitation was employed in offering the securities, the offerings and sales were made to a limited number of persons, all of whom were accredited investors and transfer was restricted by the company in accordance with the requirements of Regulation D and the Securities Act. All issuances to accredited and non-accredited investors were structured to comply with the requirements of the safe harbor afforded by Rule 506 of Regulation D, including limiting the number of non-accredited investors to no more than 35 investors who have sufficient knowledge and experience in financial and business matters to make them capable of evaluating the merits and risks of an investment in our securities.
| F-13 |
| Table of Contents |
Nine Months Ended June 30, 2022
During the nine months ended June 30, 2022, the Company issued 863,986 shares of common stock related to warrant exercises and received $793,986.
During the nine months ended June 30, 2022, the Company issued 8,750 shares related to the exercise of stock option grants and received $13,688.
The Company issued 7,672,860 shares of common stock related to the automatic conversion of Convertible Notes and interest from a private placement to accredited investors in 2021. The Convertible Notes and interested were automatically converted to Common Stock at $2.00 per share on the one year anniversary in March 2022.
On January 5, 2022, the Company issued 30,000 shares each to three directors shares at an exercise price of $1.70 per share.
Warrants to Purchase Common Stock
Nine Months Ended June 30, 2022
On January 5, 2022, the Company issued 20,000 warrants to purchase common stock each to three directors shares at $1.70 per share. The warrants expire on January 5, 2027.
During the nine months ended June 30, 2022, warrants to purchase 108,756 shares of common stock at $1.00 per share expired.
Extension of Warrant Agreement with Clayton A. Struve
On May 3, 2022, the Company approved the Extension of Warrant Agreement with Clayton Struve, extending the exercise dates as follows:
| Warrant No./Class |
| Issue Date |
| No. Warrant Shares |
|
| Exercise Price |
|
| Original Expiration Date |
| Amended Expiration Date | |||
| Clayton A. Struve Warrant |
| 08-14-2017 |
|
| 1,440,000 |
|
| $ | 0.25 |
|
| 08-13-2023 |
| 08-13-2024 | |
| Clayton A. Struve Warrant |
| 12-12-2017 |
|
| 1,200,000 |
|
| $ | 0.25 |
|
| 12-11-2023 |
| 12-11-2024 | |
| Clayton A. Struve Warrant |
| 08-04-2016 |
|
| 1,785,715 |
|
| $ | 0.25 |
|
| 08-04-2023 |
| 08-04-2024 | |
| Clayton A. Struve Warrant |
| 02-28-2018 |
|
| 1,344,000 |
|
| $ | 0.25 |
|
| 02-28-2023 |
| 02-28-2024 | |
The Company recorded interest expense of $244,260 during the nine months ended June 30, 2022 related to the extension of the warrants.
|
|
| June 30, 2022 |
|
|||||
|
|
|
|
|
| Weighted |
|
||
|
|
|
|
|
| Average |
|
||
|
|
|
|
|
| Exercise |
|
||
|
|
| Shares |
|
| Price |
|
||
| Outstanding at beginning of period |
|
| 22,564,255 |
|
| $ | 0.998 |
|
| Issued |
|
| 60,000 |
|
|
| - |
|
| Exercised |
|
| (863,986 | ) |
|
| (0.919 | ) |
| Forfeited |
|
| - |
|
|
| - |
|
| Expired |
|
| (108,756 | ) |
|
| (1.000 | ) |
| Outstanding at end of period |
|
| 21,651,513 |
|
| $ | 1.003 |
|
| Exerciseable at end of period |
|
| 21,651,513 |
|
|
|
|
|
| F-14 |
| Table of Contents |
The following table summarizes information about warrants outstanding and exercisable as of June 30, 2022:
|
|
|
| June 30, 2022 |
|
||||||||||||||
|
|
|
| Weighted |
|
| Weighted |
|
|
|
|
| Weighted |
|
|||||
|
|
|
| Average |
|
| Average |
|
|
|
|
| Average |
|
|||||
| Number of |
|
| Remaining |
|
| Exercise |
|
| Shares |
|
| Exercise |
|
|||||
| Warrants |
|
| Life ( In Years) |
|
| Price |
|
| Exerciseable |
|
| Price |
|
|||||
|
| 10,729,381 |
|
|
| 4.79 |
|
| $ | 0.250 |
|
|
| 10,729,381 |
|
| $ | 0.250 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 6,547,207 |
|
|
| 2.59 |
|
| 1.20-1.85 |
|
|
| 6,547,207 |
|
| 1.20-1.85 |
|
||
|
| 4,364,925 |
|
|
| 3.77 |
|
| 2.00-2.40 |
|
|
| 4,364,925 |
|
| 2.00-2.40 |
|
||
|
| 10,000 |
|
|
| 1.00 |
|
|
| 4.080 |
|
|
| 10,000 |
|
|
| 4.080 |
|
|
| 21,651,513 |
|
|
| 3.43 |
|
| $ | 1.003 |
|
|
| 21,651,513 |
|
| $ | 1.003 |
|
There were vested warrants of 21,651,513 with an aggregate intrinsic value of $31,557,563.
9. STOCK INCENTIVE PLANS
2021 Equity Incentive Plan
The Company initially had 20,000,000 shares of its common stock authorized as the maximum number of shares of the Company’s common stock that may be delivered to participants under the 2021 Plan, subject to adjustment for certain corporate changes affecting the shares, such as stock splits. This number was increased to 22,000,000 shares of common stock as of January 1, 2022 as a result of the automatic share reserve increase. Shares subject to an award under the 2021 Plan for which the award is canceled, forfeited or expires again become available for grants under the 2021 Plan. Shares subject to an award that is settled in cash will not again be made available for grants under the 2021 Plan. As of the date of this report on Form 10-Q, 15,722,750 shares of our common stock remain available for issuance under the 2021 Plan. The 2021 Plan also authorizes for issuance the sum of (A) any shares of our common stock that, as of the date of stockholder approval of the 2021 Plan, have been reserved but not issued pursuant to any awards granted under our 2011 Stock Incentive Plan, as amended, or the 2011 Plan, and (B) any shares of our common stock subject to stock options or similar awards granted under the 2011 Plan that, after the date of stockholder approval of the 2021 Plan, expire or otherwise terminate without having been exercised in full and shares of our common stock issued pursuant to awards granted under the 2011 Plan that are forfeited to or repurchased by us, with the maximum number of shares of our common stock to be added to the 2021 Plan pursuant to clause (ii) equal to 14,650,120.
Nine Months Ended June 30, 2022
On December 16, 2021, the Company issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On December 16, 2021, the Company issued a stock option grant to Phillip A. Bosua for 1,300,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On May 20, 2022, the Company issued a stock option grant to Peter Conley for 1,000,000 shares at an exercise price of $1.48 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years after two quarters.
During the nine months ended June 30, 2022, the Compensation committee also issued stock option grants to eighteen employees and consultants for 3,146,000 shares at an average exercise price of $1.699 per share. The stock option grants expire in five years. The stock option grant primarily vest quarterly over four years.
During the nine months ended June 30, 2022, the Company issued 8,750 shares related to the exercise of stock option grants and received $13,688.
During the nine months ended June 30, 2022, five employees and consultants forfeited stock option grants for 825,000 shares at an average $1.838 per share.
There are currently 20,927,370 (including unearned stock option grants totaling 11,550,745 shares related to performance milestones) options to purchase common stock at an average exercise price of $1.628 per share outstanding as of June 30, 2022 under the 2021 Stock Incentive Plan. The Company recorded $1,555,875 and $570,514 of compensation expense, net of related tax effects, relative to stock options for the nine months ended June 30, 2022 and 2021, respectively in accordance with ASC 718. As of June 30, 2022, there is $7,065,596, net of forfeitures, of total unrecognized costs related to employee granted stock options that are not vested. These costs are expected to be recognized over a period of approximately 4.15 years.
| F-15 |
| Table of Contents |
Stock option activity for the nine months ended June 30, 2022 and the years ended September 30, 2021 and 2020 was as follows:
|
|
| Weighted Average |
|
|||||||||
|
|
| Options |
|
| Exercise Price |
|
| Proceed $ |
|
|||
| Outstanding as of October 1, 2019 |
|
| 4,532,668 |
|
| $ | 2.025 |
|
| $ | 9,180,369 |
|
| Granted |
|
| 3,085,000 |
|
|
| 1.142 |
|
|
| 3,522,400 |
|
| Exercised |
|
| (73,191 | ) |
|
| (0.250 | ) |
|
| (18,298 | ) |
| Forfeitures |
|
| (2,739,477 | ) |
|
| (2.593 | ) |
|
| (7,103,921 | ) |
| Outstanding as of September 30, 2020 |
|
| 4,805,000 |
|
|
| 1.161 |
|
|
| 5,580,550 |
|
| Granted |
|
| 10,650,745 |
|
|
| 1.766 |
|
|
| 18,807,990 |
|
| Exercised |
|
| (20,625 | ) |
|
| (1.359 | ) |
|
| (28,031 | ) |
| Forfeitures |
|
| (120,000 | ) |
|
| (3.300 | ) |
|
| (396,000 | ) |
| Outstanding as of September 30, 2021 |
|
| 15,315,120 |
|
|
| 1.565 |
|
|
| 23,964,509 |
|
| Granted |
|
| 6,446,000 |
|
|
| 1.805 |
|
|
| 11,632,130 |
|
| Exercised |
|
| (8,750 | ) |
|
| (1.564 | ) |
|
| (13,688 | ) |
| Forfeitures |
|
| (825,000 | ) |
|
| (1.838 | ) |
|
| (1,516,150 | ) |
| Outstanding as of June 30, 2022 |
|
| 20,927,370 |
|
| $ | 1.628 |
|
| $ | 34,066,802 |
|
The following table summarizes information about stock options outstanding and exercisable as of June 30, 2022:
|
|
|
|
|
| Weighted |
|
| Weighted |
|
|
|
| Weighted |
|
||||||||
|
|
|
|
|
| Average |
|
| Average |
|
|
|
| Average |
|
||||||||
| Range of |
|
| Number |
|
| Remaining Life |
|
| Exercise Price |
|
| Number |
|
| Exercise Price |
|
||||||
| Exercise Prices |
|
| Outstanding |
|
| In Years |
|
| Outstanding |
|
| Exerciseable |
|
| Exerciseable |
|
||||||
| $ | 0.25 |
|
|
| 230,000 |
|
|
| 0.96 |
|
| $ | 0.250 |
|
|
| 201,250 |
|
| $ | 0.250 |
|
| 1.10-1.25 |
|
|
| 2,930,625 |
|
|
| 2.35 |
|
|
| 1.101 |
|
|
| 548,307 |
|
|
| 1.105 |
|
|
| 1.28-1.67 |
|
|
| 12,330,745 |
|
|
| 2.46 |
|
|
| 1.503 |
|
|
| 1,175,521 |
|
|
| 1.303 |
|
|
| 1.79-3.67 |
|
|
| 5,436,000 |
|
|
| 4.25 |
|
|
| 1.195 |
|
|
| 665,063 |
|
|
| 1.917 |
|
|
|
|
|
|
|
| 20,927,370 |
|
|
| 4.15 |
|
|
| 1.628 |
|
|
| 2,590,141 |
|
| $ | 1.241 |
|
There are stock option grants of 20,927,370 shares as of June 30, 2022 with an aggregate intrinsic value of $22,540,075.
As of September 30, 2021, the 2020 Particle Stock Incentive Plan, was terminated and all stock option grants were cancelled by the participants. The Company recorded $197,553 and $833,771 of compensation expense, net of related tax effects, relative to Particle stock options for the years ended September 30, 2021 and 2020 and in accordance with ASC 718.
10. SIGNIFICANT AND OTHER TRANSACTIONS WITH RELATED PARTIES
Transactions with Clayton Struve
See Notes 7 and 8 for related party transactions with Clayton A. Struve.
The Company owes Clayton A. Struve $1,071,000 under convertible promissory or OID notes. The Company recorded accrued interest of $84,671 and $79,062 as of June 30, 2022 and September 30, 2021, respectively. On May 3, 2022, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2022.
Related Party Transactions with Ronald P. Erickson
See Notes 7, 9, and 11 for related party transactions with Ronald P. Erickson.
On December 16, 2021, the Company issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
Mr. Erickson and/or entities with which he is affiliated also have accrued compensation, travel and interest of approximately $274,640 and $421,599 as of June 30, 2022 and September 30, 2021, respectively.
During the nine months ended June 30, 2022, the Company paid $120,000 of salaries to Mr. Erickson that were previously accrued and reported but were deferred.
Related Party Transaction with Phillip A. Bosua
See Notes 9 and 11 for related party transactions with Phillip A. Bosua.
| F-16 |
| Table of Contents |
On December 16, 2021, the Company issued a stock option grant to Phillip A. Bosua for 1,300,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
As of December 31, 2021 the Company had recorded an accounts receivable-related party of $3,124,581 for the cash it expected to receive from the CEO’s personal digital account.
The Company has established a digital wallet and corporate account at Circle in order to receive the Ethereum and then convert it to cash. The Company received $2,908,551 of Ethereum and recorded a reduction in value of $169,884 related to the decline in value of the Ethereum. The accounts receivable-related party was $46,146 as of June 30, 2022. As of June 30, 2022, accrued expenses include approximately $436,378 of expenses, primarily sales and use tax and other expenses. As of June 30, 2022 accrued expenses-related party include $799,353 of unpaid compensation that was due and payable to the CEO for the NFT revenue. This unpaid compensation was paid in July 2022 During 2021, approximately $1.3 million of the selling and transactional costs for the digital assets was paid through the CEO’s personal digital asset account including approximately $1.075 million which was paid to a consultant via the transfer of Ethereum.
Related Party Transactions with Directors
On January 5, 2022, the Company issued 30,000 shares each to three directors shares at an exercise price of $1.70 per share.
On January 5, 2022, the Company issued 20,000 warrants to purchase common stock each to three directors shares at $1.70 per share. The warrants expire on January 5, 2027.
11. COMMITMENTS, CONTINGENCIES AND LEGAL PROCEEDINGS
Legal Proceedings
The Company may from time to time become a party to various legal proceedings arising in the ordinary course of our business. The Company is currently not a party to any pending legal proceeding that is not ordinary routine litigation incidental to our business.
Employment Agreements
Phillip A. Bosua, Chief Executive Officer
On April 10, 2018, we entered into an employment agreement with Mr. Bosua reflecting his appointment as Chief Executive Officer. The employment agreement is for an initial term of 12 months (subject to earlier termination) and will be automatically extended for additional 12-month terms unless either party notifies the other party of its intention to terminate the Employment Agreement with at least ninety (90) days prior to the end of the Initial Term or renewal term. Mr. Bosua was paid a base salary of $225,000 per year, received 500,000 shares of common stock valued at $0.33 per share and may be entitled to bonuses and equity awards at the discretion of the Board or a committee of the Board. The employment agreement provides for severance pay equal to 12 months of base salary if Mr. Bosua is terminated without “cause” or voluntarily terminates his employment for “good reason.” During the years ended September 30, 2021 and 2020, our compensation committee and our board of directors compensated Mr. Bosua with an annual salary of $240,000 from October 1, 2019 to May 1, 2020. From May 1, 2020 to March 31, 2021, the annual compensation was $260,000. From April 1, 2021 to September 30, 2021, the annual compensation was $350,000. The compensation committee and the board of directors of Particle compensated Phillip A. Bosua with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. Mr. Bosua will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. If our company terminates Mr. Bosua’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Bosua terminates his employment at any time for “good reason” or due to a “disability,” Mr. Bosua will be entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months.
Peter Conley, Chief Financial Officer and Senior Vice President, Intellectual Property
On May 13, 2022, we entered into an employment agreement with Mr. Conley reflecting his appointment as our Chief Financial Officer and Senior Vice President, Intellectual Property. The employment agreement is at will, meaning either we or Mr. Conley may terminate the employment relationship at any time, with or without cause, upon written notice to the other party. Under the terms of this agreement, Mr. Conley has an annualized base salary of $300,000, and the base salary will be paid periodically in accordance with our normal payroll practice. Mr. Conley may also be entitled to bonuses from time to time as determined by our board of directors or our Compensation Committee in their sole discretion. The employment agreement provides that Mr. Conley is eligible for equity awards under our 2021 Equity Incentive Plan or by agreement outside the plan. The employment agreement provides for severance pay equal to 12 months of then-in-effect base salary if Mr. Conley is terminated without “cause” or voluntarily terminates his employment for “good reason,” as defined in the employment agreement. Mr. Conley is eligible to participate in of all our employee benefit plans, policies and arrangements that are applicable to other executive officers, as such plans, policies and arrangements may exist or change from time to time at our discretion. We will reimburse Mr. Conley for reasonable travel, entertainment and other expenses he incurs in the furtherance of his duties under this agreement.
| F-17 |
| Table of Contents |
Ronald P. Erickson, Chairman of the Board
On April 10, 2018, we entered into an amended employment agreement for Ronald P. Erickson which amends our employment agreement with him dated July 1, 2017. The initial one-year term of this amended agreement expired March 21, 2019, and automatically extends for additional one (1) year periods unless either party delivers written notice of such party’s intention to terminate the agreement at least ninety (90) days prior to the end of the renewal term. During the years ended September 30, 2021 and 2020, our compensation committee and our board of directors compensated Mr. Erickson with an annual salary of $195,000 from October 1, 2019 to May 1, 2020. From May 1, 2020 to March 31, 2021, the annual compensation was $215,000. From April 1, 2021 to September 30, 2021, the annual compensation was $300,000. The compensation committee and the board of Particle compensated Mr. Erickson with an annual salary of $120,000 from June 1, 2020 to August 15, 2021. Mr. Erickson will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. If our company terminates Mr. Erickson’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Erickson terminates his employment at any time for “good reason” or due to a “disability,” Mr. Erickson will be entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months.
Properties and Operating Leases
The Company is obligated under the following leases for its various facilities.
Corporate Offices
On April 13, 2017, the Company leased our executive office located at 500 Union Street, Suite 810, Seattle, Washington, USA, 98101. The Company leases 943 square feet and the current net monthly payment is $3,334. The monthly payment increases approximately 3% each year and the lease expires on May 31, 2022. On October 31, 2021, we extended the lease from June 1, 2022 to May 31, 2023 at $2,986 per month.
Lab Facilities and Executive Offices
On February 1, 2019, the Company leased its lab facilities and executive offices located at 915 E Pine Street, Suite 212, Seattle, WA 98122. The Company leases 2,642 square feet and the net monthly payment at September 30, 2021 is $8,697. The monthly payment increases approximately 3% annually each year on July 1. The lease expires on June 30, 2024. On October 11, 2021, the Company entered into First Amendment of Lease and added 1,030 square feet for year for $5,000 per month. The space will be utilized for clinical trials.
12. SEGMENT REPORTING
The management of the Company considers the business to currently have three operating segments (i) the development of the Bio-RFID™” and “ChromaID” technologies; (ii) Particle, Inc. technology; and (iii) AI sales of NFT products. Particle commenced operations in the three months ended June 30, 2020. AI commenced operations during the nine months ended June 30, 2022.
| F-18 |
| Table of Contents |
The reporting for the three and nine months ended June 30, 2022 and 2021 was as follows (in thousands):
|
|
|
|
|
| Segment |
|
|
|
|
|||
|
|
|
|
|
| Operating |
|
| Segment |
|
|||
| Segment |
| Revenue |
|
| (Loss) |
|
| Assets |
|
|||
| Three Months Ended June 30, 2022 |
|
|
|
|
|
|
|
|
|
|||
| Development of the Bio-RFID™” and “ChromaID™” technologies |
| $ | - |
|
| $ | (2,838 | ) |
| $ | 7,522 |
|
| Particle, Inc. technology |
|
| - |
|
|
| (23 | ) |
|
| - |
|
| Digital asset sales |
|
| - |
|
|
| (164 | ) |
|
| 2,105 |
|
| Total segments |
| $ | - |
|
| $ | (3,025 | ) |
| $ | 9,627 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Three Months Ended June 30, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
| Development of the Bio-RFID™” and “ChromaID™” technologies |
| $ | - |
|
| $ | (1,719 | ) |
| $ | 13,993 |
|
| Particle, Inc. technology |
|
| - |
|
|
| (144 | ) |
|
| 33 |
|
| Total segments |
| $ | - |
|
| $ | (1,863 | ) |
| $ | 14,026 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Segment |
|
|
|
|
|
|
|
|
|
|
|
|
| Operating |
|
| Segment |
|
||
| Segment |
| Revenue |
|
| Profit (Loss) |
|
| Assets |
|
|||
| Nine Months Ended June 30, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
| Development of the Bio-RFID™” and “ChromaID™” technologies |
| $ | - |
|
| $ | (7,616 | ) |
| $ | 7,522 |
|
| Particle, Inc. technology |
|
| - |
|
|
| (45 | ) |
|
| - |
|
| Digital asset sales |
|
| 4,360 |
|
|
| 923 |
|
|
| 2,105 |
|
| Total segments |
| $ | 4,360 |
|
| $ | (6,738 | ) |
| $ | 9,627 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Nine Months Ended June 30, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
| Development of the Bio-RFID™” and “ChromaID™” technologies |
| $ | - |
|
| $ | (7,088 | ) |
| $ | 13,993 |
|
| Particle, Inc. technology |
|
| - |
|
|
| (940 | ) |
|
| 33 |
|
| Total segments |
| $ | - |
|
| $ | (8,028 | ) |
| $ | 14,026 |
|
During the nine months ended June 30, 2022 and 2021, the Company incurred non-cash expenses related to operations of $9,242,544 and $12,203,424, respectively.
13. SUBSEQUENT EVENTS
The Company evaluated subsequent events, for the purpose of adjustment or disclosure, up through the date the financial statements were issued. Subsequent to June 30, 2022, there were the following material transactions that require disclosure:
On July 6, 2022, the Company issued a stock option grant to an employee for 150,000 shares at an exercise price of $2.24 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years after two quarters.
| F-19 |
| Table of Contents |
Report of Independent Registered Public Accounting Firm
To the Board of directors and Stockholders of
Know Labs, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Know Labs, Inc. and subsidiaries (the Company) as of September 30, 2021 and 2020, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the two years in the period ended September 30, 2021 and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2021 and 2020, and the results of its operations and its cash flows for each of the two years in the period ended September 30, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
Convertible Notes Payable
As described in Note 7 to the consolidated financial statements, the Company has entered into rounds of financing for the issuance of convertible notes with attached warrants. The convertible note agreements contain conversion and redemption features that may be considered derivative instruments. The redemption feature that may be considered a derivative instrument is the following: Mandatory Conversion on Qualified Financing. Each Holder will be required to convert the Note into a Qualified Financing at a conversion price per share equal to the lower of (i) $2.00 or (ii) a twenty-five percent (25%) discount to the price per share paid by investors in such Qualified Financing. This mandatory conversion shall be automatic and Holder will receive a number of shares based on the conversion price for the principal amount of the note and unpaid interest. The 25% discount (redemption feature) is considered a derivative liability. Management concluded that the accounting impact is negligible as the probability that a qualified financing will take place before maturity date is remote and the redemption feature value was nominal based upon the stock price as of September 30, 2021.
| F-20 |
| Table of Contents |
The principal considerations for our determination that performing procedures relating to convertible notes is a critical audit matter are the significant amount of judgement by management in developing the assumptions of the attached redemption features, which in turn led to significant auditor judgement, subjectivity, and effort in performing audit procedures and evaluating audit evidence relating to proper classification and valuation of this feature.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included analyzing the convertible note agreements, performing technical research and relying on authoritative guidance to evaluate the treatment for the redemption feature and management’s conclusion. The procedures also included, among others, evaluating and discussing with management the Company’s current liquidity position and their intentions to undertake a qualified financing prior to the maturity dates of the convertible notes, recalculating the value of the conversion and redemption features in the convertible notes based upon the stock price at September 30, 2021 and the length of time until maturity of the convertible notes.
/s/ BPM LLP
BPM LLP
We served as the Company’s auditor since October 2019
Walnut Creek, California
December 21, 2021
| F-21 |
| Table of Contents |
KNOW LABS, INC.
AUDITED CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED SEPTEMBER 30, 2021 AND 2020
KNOW LABS, INCORPORATED AND SUBSIDIARIES
|
|
| September 30, 2021 |
|
| September 30, 2020 |
|
||
| ASSETS |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||
| CURRENT ASSETS: |
|
|
|
|
|
|
||
| Cash and cash equivalents |
| $ | 12,258,218 |
|
| $ | 4,298,179 |
|
| Total current assets |
|
| 12,258,218 |
|
|
| 4,298,179 |
|
|
|
|
|
|
|
|
|
|
|
| PROPERTY AND EQUIPMENT, NET |
|
| 328,504 |
|
|
| 128,671 |
|
|
|
|
|
|
|
|
|
|
|
| OTHER ASSETS |
|
|
|
|
|
|
|
|
| Intangible assets |
|
| - |
|
|
| 101,114 |
|
| Other assets |
|
| 13,767 |
|
|
| 25,180 |
|
| Operating lease right of use asset |
|
| 289,002 |
|
|
| 129,003 |
|
|
|
|
|
|
|
|
|
|
|
| TOTAL ASSETS |
| $ | 12,889,491 |
|
| $ | 4,682,147 |
|
|
|
|
|
|
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CURRENT LIABILITIES: |
|
|
|
|
|
|
|
|
| Accounts payable - trade |
| $ | 419,093 |
|
| $ | 493,497 |
|
| Accrued expenses |
|
| 893,137 |
|
|
| 401,178 |
|
| Accrued expenses - related parties |
|
| 421,599 |
|
|
| 591,600 |
|
| Convertible notes payable, net |
|
| 9,191,155 |
|
|
| 3,967,578 |
|
| Simple Agreements for Future Equity |
|
| - |
|
|
| 785,000 |
|
| Notes payable- PPP loans, current |
|
| 431,803 |
|
|
| - |
|
| Current portion of operating lease right of use liability |
|
| 112,371 |
|
|
| 108,779 |
|
| Total current liabilities |
|
| 11,469,158 |
|
|
| 6,347,632 |
|
|
|
|
|
|
|
|
|
|
|
| NON-CURRENT LIABILITIES: |
|
|
|
|
|
|
|
|
| Notes payable- PPP loans |
|
| - |
|
|
| 226,170 |
|
| Operating lease right of use liability, net of current portion |
|
| 178,170 |
|
|
| 23,256 |
|
| Total non-current liabilities |
|
| 178,170 |
|
|
| 249,426 |
|
|
|
|
|
|
|
|
|
|
|
| COMMITMENTS AND CONTINGENCIES (Note 12) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| STOCKHOLDERS' EQUITY (DEFICIT) |
|
|
|
|
|
|
|
|
| Preferred stock - $0.001 par value, 5,000,000 shares authorized, 0 shares issued and outstanding at 9/30/2021 and 9/30/2020, respectively |
|
| - |
|
|
| - |
|
| Series C Convertible Preferred stock - $0.001 par value, 1,785,715 shares authorized, 1,785,715 shares issued and outstanding at 9/30/2021 and 9/30/2020, respectively |
|
| 1,790 |
|
|
| 1,790 |
|
| Series D Convertible Preferred stock - $0.001 par value, 1,016,014 shares authorized, 1,016,004 shares issued and outstanding at 9/30/2021 and 9/30/2020, respectively |
|
| 1,015 |
|
|
| 1,015 |
|
| Common stock - $0.001 par value, 100,000,000 shares authorized, 35,166,551 and 24,804,874 shares issued and outstanding at 9/30/2021 and 9/30/2020, respectively |
|
| 35,168 |
|
|
| 24,807 |
|
| Additional paid in capital |
|
| 82,530,684 |
|
|
| 54,023,758 |
|
| Accumulated deficit |
|
| (81,326,494 | ) |
|
| (55,966,281 | ) |
| Total stockholders' equity (deficit) |
|
| 1,242,163 |
|
|
| (1,914,911 | ) |
|
|
|
|
|
|
|
|
|
|
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) |
| $ | 12,889,491 |
|
| $ | 4,682,147 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-22 |
| Table of Contents |
KNOW LABS, INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
| Years Ended, |
|
|||||
|
|
| September 30, 2021 |
|
| September 30, 2020 |
|
||
|
|
|
|
|
|
|
|
||
| REVENUE |
| $ | - |
|
| $ | 121,939 |
|
| COST OF SALES |
|
| - |
|
|
| 69,726 |
|
| GROSS PROFIT |
|
| - |
|
|
| 52,213 |
|
| RESEARCH AND DEVELOPMENT EXPENSES |
|
| 3,969,972 |
|
|
| 2,033,726 |
|
| SELLING, GENERAL AND ADMINISTRATIVE EXPENSES |
|
| 6,476,176 |
|
|
| 4,844,415 |
|
| OPERATING LOSS |
|
| (10,446,148 | ) |
|
| (6,825,928 | ) |
|
|
|
|
|
|
|
|
|
|
| OTHER INCOME (EXPENSE): |
|
|
|
|
|
|
|
|
| Interest expense |
|
| (14,914,065 | ) |
|
| (6,094,682 | ) |
| Other income |
|
| - |
|
|
| 65,769 |
|
| (Loss) on debt settlements |
|
| - |
|
|
| (707,800 | ) |
| Total other (expense), net |
|
| (14,914,065 | ) |
|
| (6,736,713 | ) |
|
|
|
|
|
|
|
|
|
|
| LOSS BEFORE INCOME TAXES |
|
| (25,360,213 | ) |
|
| (13,562,641 | ) |
|
|
|
|
|
|
|
|
|
|
| Income tax expense |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
| NET LOSS |
| $ | (25,360,213 | ) |
| $ | (13,562,641 | ) |
|
|
|
|
|
|
|
|
|
|
| Basic and diluted loss per share |
| $ | (0.86 | ) |
| $ | (0.62 | ) |
|
|
|
|
|
|
|
|
|
|
| Weighted average shares of common stock outstanding- basic and diluted |
|
| 29,370,596 |
|
|
| 21,791,058 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-23 |
| Table of Contents |
KNOW LABS, INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
| Series C Convertible |
|
| Series D Convertible |
|
|
|
|
|
| Additional |
|
|
|
| Total Stockholders' |
|
||||||||||||||||||
|
|
| Preferred Stock |
|
| Preferred Stock |
|
| Common Stock |
|
| Paid in |
|
| Accumulated |
|
| Equity |
|
||||||||||||||||||
|
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| (Deficit) |
|
|||||||||
| Balance as of September 30, 2019 |
|
| 1,785,715 |
|
| $ | 1,790 |
|
|
| 1,016,004 |
|
| $ | 1,015 |
|
|
| 18,366,178 |
|
| $ | 18,366 |
|
| $ | 39,085,179 |
|
| $ | (42,403,640 | ) |
| $ | (3,297,290 | ) |
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,702,085 |
|
|
| - |
|
|
| 1,702,085 |
|
| Stock option exercise |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 73,191 |
|
|
| 73 |
|
|
| (73 | ) |
|
| - |
|
|
| - |
|
| Conversion of debt offering and accrued interest (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 4,581,917 |
|
|
| 4,585 |
|
|
| 4,591,952 |
|
|
| - |
|
|
| 4,596,537 |
|
| Beneficial conversion feature (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 3,766,074 |
|
|
| - |
|
|
| 3,766,074 |
|
| Issuance of warrants to debt holders (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,824,998 |
|
|
| - |
|
|
| 1,824,998 |
|
| Issuance of warrants for services related to debt offering (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 975,326 |
|
|
| - |
|
|
| 975,326 |
|
| Issuance of common stock for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 550,000 |
|
|
| 550 |
|
|
| 1,044,450 |
|
|
| - |
|
|
| 1,045,000 |
|
| Issuance of common stock for exercise of warrants |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 733,588 |
|
|
| 733 |
|
|
| 84,267 |
|
|
| - |
|
|
| 85,000 |
|
| Issuance of shares related to Settlement and Mutual Release and Subscription Agreements |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 500,000 |
|
|
| 500 |
|
|
| 949,500 |
|
|
| - |
|
|
| 950,000 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (13,562,641 | ) |
|
| (13,562,641 | ) |
| Balance as of October 1, 2020 |
|
| 1,785,715 |
|
|
| 1,790 |
|
|
| 1,016,004 |
|
|
| 1,015 |
|
|
| 24,804,874 |
|
|
| 24,807 |
|
|
| 54,023,758 |
|
|
| (55,966,281 | ) |
|
| (1,914,911 | ) |
| Stock compensation expense - employee options |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,028,522 |
|
|
| - |
|
|
| 1,028,522 |
|
| Conversion of debt offering and accrued interest (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 6,090,660 |
|
|
| 6,091 |
|
|
| 6,091,968 |
|
|
| - |
|
|
| 6,098,058 |
|
| Beneficial conversion feature (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 9,769,683 |
|
|
| - |
|
|
| 9,769,683 |
|
| Issuance of warrants to debt holders (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 4,439,317 |
|
|
| - |
|
|
| 4,439,317 |
|
| Issuance of warrants for services related to debt offering (Note 7) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,667,281 |
|
|
| - |
|
|
| 1,667,281 |
|
| Issuance of common stock for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 97,000 |
|
|
| 97 |
|
|
| 202,723 |
|
|
| - |
|
|
| 202,820 |
|
| Issuance of warrant for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 2,547,436 |
|
|
| - |
|
|
| 2,547,436 |
|
| Issuance of common stock for exercise of warrants |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 3,676,542 |
|
|
| 3,675 |
|
|
| 1,309,528 |
|
|
| - |
|
|
| 1,313,203 |
|
| Issuance of common stock for stock option exercises |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 16,875 |
|
|
| 17 |
|
|
| 23,327 |
|
|
| - |
|
|
| 23,344 |
|
| Issuance of shares and warrants for conversion of Simple Agreements for Future Equity |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 480,600 |
|
|
| 481 |
|
|
| 1,427,141 |
|
|
| - |
|
|
| 1,427,622 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (25,360,213 | ) |
|
| (25,360,213 | ) |
| Balance as of September 30, 2021 |
|
| 1,785,715 |
|
| $ | 1,790 |
|
|
| 1,016,004 |
|
| $ | 1,015 |
|
|
| 35,166,551 |
|
| $ | 35,168 |
|
| $ | 82,530,684 |
|
| $ | (81,326,494 | ) |
| $ | 1,242,163 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-24 |
| Table of Contents |
KNOW LABS, INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
| Years Ended, |
|
||||||
|
|
|
| September 30, 2021 |
|
| September 30, 2020 |
|
|||
|
|
|
|
|
|
|
|
|
|||
| CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|||
| Net loss |
|
| $ | (25,360,213 | ) |
| $ | (13,562,641 | ) | |
| Adjustments to reconcile net loss to net cash (used in) operating activities |
|
|
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
|
| 200,807 |
|
|
| 242,987 |
|
|
| Issuance of capital stock for services and expenses |
|
|
| 202,820 |
|
|
| 1,045,000 |
|
|
| Stock based compensation- warrants |
|
|
| 2,547,436 |
|
|
| - |
|
|
| Stock based compensation- stock option grants |
|
|
| 1,028,522 |
|
|
| 1,702,085 |
|
|
| Amortization of debt discount to interest expense |
|
|
| 13,722,672 |
|
|
| 5,662,690 |
|
|
| Right of use, net |
|
|
| (1,493 | ) |
|
| 422 |
|
|
| Loss on sale of assets |
|
|
| - |
|
|
| 4,663 |
|
|
| Loss (gain) on debt settlement |
|
|
| - |
|
|
| (117,200 | ) | |
| Loss related to issuance of shares for debt settlement |
|
|
| - |
|
|
| 825,000 |
|
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
| Accounts receivable |
|
|
| - |
|
|
| 63,049 |
|
|
| Prepaid expenses |
|
|
| - |
|
|
| 6,435 |
|
|
| Inventory |
|
|
| - |
|
|
| 7,103 |
|
|
| Other long-term assets |
|
|
| 11,413 |
|
|
| (11,414 | ) | |
| Accounts payable - trade and accrued expenses |
|
|
| 797,337 |
|
|
| 218,018 |
|
|
| NET CASH (USED IN) OPERATING ACTIVITIES |
|
|
| (6,850,699 | ) |
|
| (3,913,803 | ) | |
|
|
|
|
|
|
|
|
|
|
|
|
| CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
| Purchase of research and development equipment |
|
|
| (299,525 | ) |
|
| (70,134 | ) | |
| NET CASH (USED IN) INVESTING ACTIVITIES: |
|
|
| (299,525 | ) |
|
| (70,134 | ) | |
|
|
|
|
|
|
|
|
|
|
|
|
| CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
| Proceeds from convertible notes payable |
|
|
| 14,209,000 |
|
|
| 5,639,500 |
|
|
| Payments for issuance costs from notes payable |
|
|
| (727,117 | ) |
|
| (479,965 | ) | |
| Proceeds from Simple Agreements for Future Equity |
|
|
| 340,000 |
|
|
| 785,000 |
|
|
| Repayments on Simple Agreements for Future Equity |
|
|
| (253,800 | ) |
|
| - |
|
|
| Proceeds from note payable - PPP |
|
|
| 205,633 |
|
|
| 226,170 |
|
|
| Proceeds from issuance of common stock for stock options exercise |
|
|
| 23,344 |
|
|
| - |
|
|
| Proceeds from issuance of common stock for warrant exercise |
|
|
| 1,313,203 |
|
|
| 85,575 |
|
|
| Proceeds from issuance of shares related to debt settlement |
|
|
| - |
|
|
| 125,000 |
|
|
| NET CASH PROVIDED BY FINANCING ACTIVITIES |
|
|
| 15,110,263 |
|
|
| 6,381,280 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NET INCREASE N CASH AND CASH EQUIVALENTS |
|
|
| 7,960,039 |
|
|
| 2,397,343 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CASH AND CASH EQUIVALENTS, beginning of period |
|
|
| 4,298,179 |
|
|
| 1,900,836 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CASH AND CASH EQUIVALENTS, end of period |
|
| $ | 12,258,218 |
|
| $ | 4,298,179 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
|
| Interest paid |
|
| $ | 18,800 |
|
| $ | - |
|
|
| Taxes paid |
|
| $ | - |
|
| $ | 1,922 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
| $ | 9,769,683 |
|
| $ | 3,766,074 |
|
|
| Issuance of warrants to debt holders |
|
| $ | 4,439,317 |
|
| $ | 1,824,998 |
|
|
| Issuance of warrants for services related to debt offering |
|
| $ | 1,667,281 |
|
| $ | 975,326 |
|
|
| Cashless warrant exercise (fair value) |
|
| $ | 515,975 |
|
| $ | 111,554 |
|
|
| Cashless stock options exercise (fair value) |
|
| $ | - |
|
| $ | 18,298 |
|
|
| Conversion of debt offering |
|
| $ | 5,638,275 |
|
| $ | 4,245,448 |
|
|
| Conversion of accrued interest |
|
| $ | 460,185 |
|
| $ | 351,089 |
|
|
| Issuance of shares and warrants for conversion of Simple Agreements for Future Equity |
|
| $ | 1,427,141 |
|
| $ | - |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-25 |
| Table of Contents |
KNOW LABS, INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION
Know Labs, Inc. (the “Company”) was incorporated under the laws of the State of Nevada in 1998. The Company has authorized 105,000,000 shares of capital stock, of which 100,000,000 are shares of voting common stock, par value 0.001 per share, and 5,000,000 are shares preferred stock, par value $0.001 per share. At the annual shareholder meeting held on October 15, 2021, our authorized shares of common stock was increased to 200,000,000 shares of voting common stock, par value $0.001 per share.
The Company is focused on the development and commercialization of proprietary biosensor technologies which, when paired with our AI deep learning platform, are capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify and measure the unique “signature” of said materials or analytes. The Company calls these our “Bio-RFID™” technology platform when pertaining to radio and microwave spectroscopy; and “ChromaID” technology platform when pertaining to optical spectroscopy. The data obtained with the Company’s biosensor technology is analyzed with our trade secret algorithms which are driven by our AI Deep Learning platform.
ChromaID is the first technology developed and patented by the Company. For the past several years, the Company has focused upon extensions and new patentable inventions that are derived from and extend beyond our ChromaID technology and intellectual property. The Company calls this technology platform Bio-RFID. The rapid advances made with our Bio-RFID technology in our laboratory have caused us to move quickly into the commercialization phase of our Company as we work to create revenue generating products for the marketplace. Today, the primary focus of the Company is on its Bio-RFID technology, its commercialization and development of related patent assets. Through its wholly owned subsidiary corporations the Company works to exploit additional opportunities and markets that its broad intellectual property and trade secret portfolio addresses.
On April 30, 2020, the Company approved and ratified the incorporation of Particle, Inc. Particle is focused on the development and commercialization of our extensive intellectual property relating to electromagnetic energy outside of the medical diagnostic arena which remains the parent company’s singular focus. Since incorporation, Particle has engaged in research and development activities on threaded light bulbs that have a warm white light and can inactivate germs, including bacteria and viruses. It is now looking for partners to take the product to market.
On September 17, 2021, the Company approved and ratified the incorporation of AI Mind, Inc. AI Mind is focused on monetizing the AI Deep Learning Platform. Since incorporation it has focused on creating patterns from Company data which were sold as NFTs. The Company will continue to look for opportunities for new applications on its AI Deep Learning Platform to generate revenues to support the continued development of its non-invasive diagnostic technology.
In 2010, the Company acquired TransTech Systems, Inc. as an adjunct to the Company’s business. TransTech was a distributor of products for employee and personnel identification and authentication. TransTech historically provided substantially all of the Company’s revenues. The financial results from our TransTech subsidiary had been diminishing as vendors of their products increasingly moved to the Internet and direct sales to their customers. While it did provide our prior year revenues, it was not central to our current focus as a Company. Moreover, the Company wrote down any goodwill associated with its historic acquisition. TransTech ceased operation on June 30, 2020 and was dissolved as of September 30, 2020.
2. LIQUIDITY
The Company has cash of approximately $12,258,218 and net working capital of approximately $10,092,586 (exclusive of convertible notes payable and right of use asset and liabilities) as of September 30, 2021.The Company anticipates that it will record losses from operations for the foreseeable future. The Company believes that it has enough available cash to operate until December 2023. As of September 30, 2021, the Company’s accumulated deficit was $81,326,494. The Company has had limited capital resources and intends to seek additional cash via equity and debt offerings. .
On March 15, 2021, the Company closed private placement for gross proceeds of $14,209,000 in exchange for issuing 8% Subordinated Convertible Notes and 3,552,250 Warrants in a private placement to accredited investors, pursuant to a series of substantially identical Securities Purchase Agreements, Common Stock Warrants, and related documents. The Convertible Notes and accrued interest will be automatically converted to Common Stock at $2.00 per share on the one year anniversary starting on March 15, 2022.
See Note 15 for discussion of transactions subsequent to September 30, 2021 that generated additional cash for the Company.
| F-26 |
| Table of Contents |
3. SIGNIFICANT ACCOUNTING POLICIES: ADOPTION OF ACCOUNTING STANDARDS
Basis of Presentation – The accompanying consolidated financial statements include the accounts of the Company. Intercompany accounts and transactions have been eliminated. The preparation of these unaudited condensed consolidated financial statements were prepared in conformity with U.S. generally accepted accounting principles (“GAAP”).
Principles of Consolidation – The consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, TransTech Systems, Inc. and RAAI Lighting, Inc., and Particle, Inc. Inter-Company items and transactions have been eliminated in consolidation.
Cash and Cash Equivalents – The Company classifies highly liquid temporary investments with an original maturity of three months or less when purchased as cash equivalents. The Company maintains cash balances at various financial institutions. Balances at US banks are insured by the Federal Deposit Insurance Corporation up to $250,000. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risk for cash on deposit. At September 30, 2021 and 2020, the Company had uninsured deposits in the amount of $12,008,228 and $4,048,179, respectively.
Equipment – Equipment consists of machinery, leasehold improvements, furniture and fixtures and software, which are stated at cost less accumulated depreciation and amortization. Depreciation is computed by the straight-line method over the estimated useful lives or lease period of the relevant asset, generally 2-5 years, except for leasehold improvements which are depreciated over 5 years.
Long-Lived Assets – The Company reviews its long-lived assets for impairment annually or when changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Long-lived assets under certain circumstances are reported at the lower of carrying amount or fair value. Assets to be disposed of and assets not expected to provide any future service potential to the Company are recorded at the lower of carrying amount or fair value (less the projected cost associated with selling the asset). To the extent carrying values exceed fair values, an impairment loss is recognized in operating results.
Intangible Assets – Intangible assets are capitalized and amortized on a straight-line basis over their estimated useful life, if the life is determinable. If the life is not determinable, amortization is not recorded. We regularly perform reviews to determine if facts and circumstances exist which indicate that the useful lives of our intangible assets are shorter than originally estimated or the carrying amount of these assets may not be recoverable. When an indication exists that the carrying amount of intangible assets may not be recoverable, we assess the recoverability of our assets by comparing the projected undiscounted net cash flows associated with the related asset or group of assets over their remaining lives against their respective carrying amounts. Such impairment test is based on the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. Impairment, if any, is based on the excess of the carrying amount over the estimated fair value of those assets.
Research and Development Expenses – Research and development expenses consist of the cost of employees, consultants and contractors who design, engineer and develop new products and processes as well as materials, supplies and facilities used in producing prototypes.
The Company’s current research and development efforts are primarily focused on improving our Bio-RFID technology, extending its capacity and developing new and unique applications for this technology. As part of this effort, the Company conducts on-going laboratory testing to ensure that application methods are compatible with the end-user and regulatory requirements, and that they can be implemented in a cost-effective manner. The Company also is actively involved in identifying new applications. The Company’s current internal team along with outside consultants has considerable experience working with the application of the Company’s technologies and their applications. The Company engages third party experts as required to supplement our internal team. The Company believes that continued development of new and enhanced technologies is essential to our future success. The Company incurred expenses of $3,969,972 and $2,033,726 for the year ended September 30, 2021 and 2020, respectively, on development activities.
Advertising – Advertising costs are charged to selling, general and administrative expenses as incurred. Advertising and marketing costs for the years ended September 30, 2021 and 2020 were $329,375 and $230,844, respectively.
Fair Value Measurements and Financial Instruments – ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
| Level 1 – Quoted prices in active markets for identical assets and liabilities; |
| Level 2 – Inputs other than level one inputs that are either directly or indirectly observable; and.
Level 3 - Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
| F-27 |
| Table of Contents |
The recorded value of other financial assets and liabilities, which consist primarily of cash and cash equivalents, accounts receivable, other current assets, and accounts payable and accrued expenses approximate the fair value of the respective assets and liabilities as of September 30, 2021 and 2020 are based upon the short-term nature of the assets and liabilities.
The Company has a money market account which is considered a level 1 asset. The balance as of September 30, 2021 and 2020 was $12,217,714 and $4,252,959, respectively.
Derivative Financial Instruments –Pursuant to ASC 815 “Derivatives and Hedging”, the Company evaluates all of its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. The Company then determines if embedded derivative must be bifurcated and separately accounted for. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. For stock-based derivative financial instruments, the Company uses a Black-Scholes-Merton option pricing model to value the derivative instruments at inception and on subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within twelve months of the balance sheet date.
The Company determined that the conversion features for purposes of bifurcation within its currently outstanding convertible notes payable were immaterial and there was no derivative liability to be recorded as of September 30, 2021 and 2020.
Stock Based Compensation - The Company has share-based compensation plans under which employees, consultants, suppliers and directors may be granted restricted stock, as well as options and warrants to purchase shares of Company common stock at the fair market value at the time of grant. Stock-based compensation cost to employees is measured by the Company at the grant date, based on the fair value of the award, over the requisite service period under ASC 718. For options issued to employees, the Company recognizes stock compensation costs utilizing the fair value methodology over the related period of benefit.
Convertible Securities – Based upon ASC 815-15, we have adopted a sequencing approach regarding the application of ASC 815-40 to convertible securities. We will evaluate our contracts based upon the earliest issuance date. In the event partial reclassification of contracts subject to ASC 815-40-25 is necessary, due to our inability to demonstrate we have sufficient shares authorized and unissued, shares will be allocated on the basis of issuance date, with the earliest issuance date receiving first allocation of shares. If a reclassification of an instrument were required, it would result in the instrument issued latest being reclassified first.
Net Loss per Share – Under the provisions of ASC 260, “Earnings Per Share,” basic loss per common share is computed by dividing net loss available to common stockholders by the weighted average number of shares of common stock outstanding for the periods presented. Diluted net loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock. As of September 30, 2021, the Company had 35,166,551 shares of common stock issued and outstanding. As of September 30, 2021, there were options outstanding for the purchase of 15,315,120 common shares (including unearned stock option grants totaling 11,775,745 shares related to performance targets), warrants for the purchase of 22,564,255 common shares, and 8,108,356 shares of the Company’s common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock. In addition, the Company currently has 16,124,764 common shares (9,020,264 common shares at the current price of $0.25 per share and 7,104,500 common shares at the current price of $2.00 per share) reserved and are issuable upon conversion of convertible debentures of $16,464,066. All of which could potentially dilute future earnings per share but are excluded from the September 30, 2021, calculation of net loss per share because their impact is antidilutive.
As of September 30, 2020, there were options outstanding for the purchase of 4,805,000 common shares (including unearned stock option grants totaling 2,630,000 shares related to performance targets), warrants for the purchase of 20,016,367common shares, and 8,108,356 shares of the Company’s common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock. In addition, the Company currently has 14,659,764 common shares (9,020,264 common shares at the current price of $0.25 per share and 5,639,500 common shares at the current price of $1.00 per share) and are issuable upon conversion of convertible debentures of $7,894,566. All of which could potentially dilute future earnings per share but excluded from the September 30, 2020 calculation of net loss per share because their impact is antidilutive.
Comprehensive loss – Comprehensive loss is defined as the change in equity of a business during a period from non-owner sources. There were no differences between net loss for the years ended September 30, 2021 and 2020 and comprehensive loss for those periods.
Dividend Policy – The Company has never paid any cash dividends and intends, for the foreseeable future, to retain any future earnings for the development of our business. Our future dividend policy will be determined by the board of directors on the basis of various factors, including our results of operations, financial condition, capital requirements and investment opportunities.
| F-28 |
| Table of Contents |
Use of Estimates – The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Recent Accounting Pronouncements
In August 2020, the FASB issued Accounting Standards Update (“ASU”) No. 2020-06, Debt --debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging --Contracts in Entity’ Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’ Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for the derivative scope exception, and it simplifies the diluted earnings per share calculation in certain areas. The new standard is effective for the Company on October 1, 2021. Adoption of the ASU is not expected to impact the Company’s financial position, results of operations or cash flows.
Based on the Company’s review of accounting standard updates issued since the filing of the 2021 Form 10-K, there have been no other newly issued or newly applicable accounting pronouncements that have had, or are expected to have, a significant impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
4. FIXED ASSETS
Property and equipment as of September 30, 2021 and 2020 was comprised of the following:
|
|
| Estimated Useful Lives |
| September 30, 2021 |
|
| September 30, 2020 |
|
||
| Machinery and equipment |
| 2-3 years |
| $ | 654,798 |
|
| $ | 355,272 |
|
| Leasehold improvements |
| 5 years |
|
| 3,612 |
|
|
| 3,612 |
|
| Furniture and fixtures |
| 5 years |
|
| 26,855 |
|
|
| 26,855 |
|
| Less: accumulated depreciation |
|
|
|
| (356,761 | ) |
|
| (257,068 | ) |
|
|
|
|
| $ | 328,504 |
|
| $ | 128,671 |
|
Total depreciation expense was $99,693 and $69,655 for the year ended September 30, 2021 and 2020, respectively. All equipment is used for general and administrative purposes and accordingly all depreciation is classified in general and administrative expenses.
5. INTANGIBLE ASSETS
Intangible assets as of September 30, 2021 and 2020 consisted of the following:
|
|
| Estimated |
| September 30, |
|
| September 30, |
|
||
|
|
| Useful Lives |
| 2021 |
|
| 2020 |
|
||
|
|
|
|
|
|
|
|
|
|
||
| Technology |
| 3 years |
| $ | 520,000 |
|
| $ | 520,000 |
|
| Less: accumulated amortization |
|
|
|
| (520,000 | ) |
|
| (418,886 | ) |
| Intangible assets, net |
|
|
| $ | - |
|
| $ | 101,114 |
|
Total amortization expense was $101,114 and $173,332 for the years ended September 30, 2021 and 2020, respectively.
Merger with RAAI Lighting, Inc.
On April 10, 2018, the Company entered into an Agreement and Plan of Merger with 500 Union Corporation, a Delaware corporation and a wholly owned subsidiary of the Company, and RAAI Lighting, Inc., a Delaware corporation. Pursuant to the Merger Agreement, the Company acquired all the outstanding shares of RAAI’s capital stock through a merger of Merger Sub with and into RAAI (the “Merger”), with RAAI surviving the Merger as a wholly owned subsidiary of the Company.
The fair value of the intellectual property associated with the assets acquired was $520,000 estimated by using a discounted cash flow approach based on future economic benefits. In summary, the estimate was based on a projected income approach and related discounted cash flows over five years, with applicable risk factors assigned to assumptions in the forecasted results.
| F-29 |
| Table of Contents |
6. LEASES
The Company has entered into operating leases for office and development facilities. These leases have terms which range from two to three years and include options to renew. These operating leases are listed as separate line items on the Company’s September 30, 2021 and 2020 Consolidated Balance Sheets and represent the Company’s right to use the underlying asset for the lease term. The Company’s obligation to make lease payments are also listed as separate line items on the Company’s September 30, 2021 and 2020 Consolidated Balance Sheets. Based on the present value of the lease payments for the remaining lease term of the Company’s existing leases, the Company recognized right-of-use assets and lease liabilities for operating leases of approximately $291,000 as of September 30, 2021. Operating lease right-of-use assets and liabilities commencing after October 1, 2018 are recognized at commencement date based on the present value of lease payments over the lease term. During years ended September 30, 2021 and 2020, the Company had three leases expire and recognized the rent payments as an expense in the current period. As of September 30, 2021 and September 30, 2020, total operating lease liabilities for remaining long term lease was approximately $290,000 and $132,000, respectively. In the year ended September 30, 2021 and 2020, the Company recognized approximately $139,643 and $136,718, respectively in total lease costs for the leases.
Because the rate implicit in each lease is not readily determinable, the Company uses its incremental borrowing rate to determine the present value of the lease payments.
Information related to the Company’s operating right-of-use assets and related lease liabilities as of and for the year ended September 30, 2021 was as follows:
Cash paid for ROU operating lease liability $139,643
Weighted-average remaining lease term 28 months
Weighted-average discount rate 7%
The minimum future lease payments as of September 30, 2021 are as follows:
| Years Ended September 30, |
| $ |
|
|
| 2022 |
| $ | 128,987 |
|
| 2023 |
|
| 107,662 |
|
| 2024 |
|
| 73,095 |
|
| 2025 |
|
| - |
|
| 2026 |
|
| - |
|
| Total Remaining Payments |
|
| 309,744 |
|
| Of which Imputed Interest |
|
| (19,203 | ) |
| Total Lease Liability |
| $ | 290,541 |
|
7. CONVERTIBLE NOTES PAYABLE AND NOTE PAYABLE
Convertible notes payable as of September 30, 2021 and 2020 consisted of the following:
Convertible Promissory Notes with Clayton A. Struve
The Company owes Clayton A. Struve $1,071,000 under convertible promissory or OID notes. The Company recorded accrued interest of $79,062 and $71,562 as of September 30, 2021 and 2020, respectively. On December 23, 2020, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to March 31, 2021. On November 8, 2021, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to March 31, 2022.
Mr. Struve also invested $1,000,000 in the May 2019 Convertible Debt Offering and such note was converted to common stock in May 2020.
Convertible Redeemable Promissory Notes with Ronald P. Erickson and J3E2A2Z
On March 16, 2018, the Company entered into a Note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 currently owing under the J3E2A2Z Notes was converted to a Convertible Redeemable Promissory Note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z Account Payable was converted into a Convertible Redeemable Promissory Note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of common stock of the Company for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. The warrants were valued at $110,545. Because the note is immediately convertible, the warrants and beneficial conversion were expensed as interest. The Company recorded accrued interest of $216,246 and $145,202 as of September 30, 2021 and 2020, respectively. On September 30, 2021, the Company approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to March 31, 2022.
| F-30 |
| Table of Contents |
Convertible Debt Offering
Beginning in 2019, the Company entered into series of debt offerings with similar and consistent terms. The Company issued Subordinated Convertible Notes and Warrants in a private placement to accredited investors, pursuant to a series of substantially identical Securities Purchase Agreements, Common Stock Warrants, and related documents. The notes are convertible into one share of common stock for each dollar invested in a Convertible Note Payable and automatically convert to common stock after one year. The convertible notes contain terms and conditions which are deemed to be a Beneficial Conversion Feature (BCF). Warrants are issued to purchase common stock with exercise prices of $1.20 and $2.40 per share and the number of warrants are equal to 50% of the convertible note balance. The Company compensates the placement agent with a cash fee and warrants. Through December 31, 2021, the Company has raised approximately $24 million through these offerings, of which $14,209,000 and $5,639,500 were raised in the years ended September 30, 2021 and 2020, respectively.
The Convertible Notes issued during the year ended September 30, 2021 are initially convertible into 7,104,500 shares of Common Stock, subject to certain adjustments, and the Warrants are initially exercisable for 3,552,250 shares of Common Stock.
The fair value of the Warrants issued to debt holders during the year ended September 30, 2021 was $4,439,317 on the date of issuance and will be amortized over the one-year term of the Convertible Notes. The fair value of the warrants was recorded as debt discount (with an offset to APIC) and will be amortized over the one-year term of the Convertible Notes.
In connection with the debt offering during the year ended June 30, 2021, the placement agent for the Convertible Notes and the Warrants received a cash fee of $727,117 and warrants to purchase 492,090 shares of the Company’s common stock, all based on 2-8% of gross proceeds to the Company. The warrants issued for these services had a fair value of $1,667,281 at the date of issuance. The fair value of the warrants was recorded as debt discount (with an offset to APIC) and will be amortized over the one-year term of the Convertible Notes. The $727,117 cash fee was recorded as issuance costs and will be amortized over the one-year term of the related Convertible Notes.
During the years ended September 30, 2021 and 2020, the Company recorded a debt discount of $9,769,683 and $3,766,074 associated with a beneficial conversion feature on the debt, which is being accreted using the effective interest method over the one-year term of the Convertible Notes.
During the year ended September 30, 2021, the Company issued 6,091,960 shares of common stock related to the automatic conversion of Convertible Notes and interest from a private placement to accredited investors in 2020. The Convertible Notes and interested were automatically converted to Common Stock at $1.00 per share on the one year anniversary starting on October 17, 2020.
During the year ended September 30, 2021 and 2020, amortization related to the debt offerings of $13,256,250 and $5,662,690 of the beneficial conversion feature, warrants issued to debt holders and placement agent was recognized as interest expense in the consolidated statements of operations.
Convertible notes payable as of September 30, 2021 and 2020 are summarized below:
|
|
| September 30, 2021 |
|
| September 30, 2020 |
|
||
| Convertible note- Clayton A. Struve |
| $ | 1,071,000 |
|
| $ | 1,071,000 |
|
| Convertible note- Ronald P. Erickson and affiliates |
|
| 1,184,066 |
|
|
| 1,184,066 |
|
| 2019 Convertible notes |
|
| 4,242,490 |
|
|
| 4,242,490 |
|
| 2020 Convertible notes |
|
| 5,639,500 |
|
|
| 5,639,500 |
|
| Q2 2021 Convertible notes |
|
| 14,209,000 |
|
|
| - |
|
| Boustead fee refund (originally booked as contra debt) |
|
| 50,000 |
|
|
| 50,000 |
|
| Less conversions of notes |
|
| (9,881,990 | ) |
|
| (4,242,490 | ) |
| Less debt discount - BCF |
|
| (4,308,337 | ) |
|
| (2,127,894 | ) |
| Less debt discount - warrants |
|
| (1,957,590 | ) |
|
| (1,025,512 | ) |
| Less debt discount - warrants issued for services |
|
| (1,056,984 | ) |
|
| (823,582 | ) |
|
|
| $ | 9,191,155 |
|
| $ | 3,967,578 |
|
Note Payable-PPP Loans
On April 30, 2020, the Company received $226,170 under the Paycheck Protection Program of the U.S. Small Business Administration’s 7(a) Loan Program pursuant to the Coronavirus, Aid, Relief and Economic Security Act (CARES Act), Pub. Law 116-136, 134 Stat. 281 (2020). As of September 30, 2021 and 2020, the Company recorded interest expense of $3,222 and $960, respectively. The Company utilized the funds in accordance with the legal requirements and expects this loan to be forgiven. Until the loan is legally forgiven, the loan balance will outstanding. The Company filed the application for the loan forgiveness during the three months ended December 31, 2021 and the Company is expecting approval by the SBA.
| F-31 |
| Table of Contents |
On February 1, 2021, the Company received $205,633 under the Paycheck Protection Program of the U.S. Small Business Administration’s 7(a) Loan Program pursuant to the Coronavirus, Aid, Relief and Economic Security Act (CARES Act), Pub. Law 116-136, 134 Stat. 281 (2020). As of September 30, 2021, the Company recorded interest expense of $1,268. The Company utilized the funds in accordance with the legal requirements and expects this loan to be forgiven. Until the loan is legally forgiven, the loan balance will be outstanding. The Company filed the application for the loan forgiveness during the three months ended December 31, 2021 and the Company is expecting approval by the SBA.
8. SIMPLE AGREEMENTS FOR FUTURE EQUITY
In July 2020, Particle entered into Simple Agreements for Future Equity (“SAFE”) with twenty two accredited investors pursuant to which Particle received $785,000 in cash in exchange for the providing the investor the right to receive shares of the Particle stock. The Company expects to issue 981,250 shares of the Particle stock that was initially valued at $0.80 per share. The Company paid $47,100 in broker fees which were expensed as business development expenses.
In October 2020, Particle entered into Simple Agreements for Future Equity (“SAFE”) with two accredited investors pursuant to which Particle received $55,000 in cash in exchange for the providing the investor the right to receive shares of the Particle stock. The Company expects to issue 68,750 shares of the Particle stock that was initially valued at $0.80 per share. The Company paid $4,125 in broker fees which were expensed as business development expenses.
During the three months ended March 31, 2021, Particle entered into Simple Agreements for Future Equity (“SAFE”) with five accredited investors pursuant to which Particle received $340,000 in cash in exchange for the providing the investor the right to receive shares of the Particle stock. The Company expects to issue 68,750 shares of the Particle stock that was initially valued at $0.80 per share. The Company paid $23,660 in broker fees which were expensed as business development expenses.
Through August 9, 2021, $1,125,000 has been raised through the sale of SAFE instruments. On this date, certain investors elected to convert their SAFE instruments balance and accrued interest into Know Labs common stock. The Company issued 480,600 shares of common stock at an average price of $2.00 per share or $961,200 related to the conversion into the Company’s common shares. The exercise price was below the fair market value of Know Labs stock, as such the Company recorded a beneficial conversion feature of $72,090.
The Company also issued five year warrants to these investors for 240,000 shares of the Company’s common stock. The warrants are exercisable at $2.40 per share. The warrants were valued at $1.641 per share or $394,332 and were expensed during the current year The Company repaid $253,800 to investors that elected to redeem their SAFE instruments for cash.
The Company recorded interest expense of $90,000 and paid $54,108 to Boustead Securities LLC in fees during the year ended
September 30, 2021 related to this transaction. The Company also issued a five year warrant to Boustead Securities LLC for 43,254 shares of the Company’s common stock. The warrant is exercisable at $2.40 per share and was valued at $1.641 per share or $70,980.
9. EQUITY
Authorized Capital Stock
The Company has authorized 105,000,000 shares of capital stock, of which 100,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are shares of preferred stock, par value $0.001 per share. At the annual shareholder meeting held on October 15, 2021, our authorized shares of common stock was increased to 200,000,000 shares of voting common stock, par value $0.001 per share. We have authorized 5,000,000 are shares of preferred stock, par value $0.001 per share.
As of September 30, 2021, we had 35,166,551 shares of common stock issued and outstanding, held by 163 stockholders of record. The number of stockholders, including beneficial owners holding shares through nominee names, is approximately 3,600. Each share of common stock entitles its holder to one vote on each matter submitted to the stockholders for a vote, and no cumulative voting for directors is permitted. Stockholders do not have any preemptive rights to acquire additional securities issued by the Company. As of September 30, 2021, there were options outstanding for the purchase of 15,315,120 common shares (including unearned stock option grants totaling 11,775,745 shares related to performance targets), warrants for the purchase of 22,564,255 common shares, and 8,108,356 shares of the Company’s common stock issuable upon the conversion of Series C and Series D Convertible Preferred Stock. In addition, the Company currently has 16,124,764 common shares (9,020,264 common shares at the current price of $0.25 per share and 7,104,500 common shares at the current price of $2.00 per share) reserved and are issuable upon conversion of convertible debentures of $16,464,066. All of which could potentially dilute future earnings per share but are excluded from the September 30, 2021, calculation of net loss per share because their impact is antidilutive.
| F-32 |
| Table of Contents |
Series C and D Preferred Stock and Warrants
On August 5, 2016, the Company closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve, an accredited investor for the purchase of $1,250,000 of preferred stock with a conversion price of $0.70 per share. The preferred stock has a yield of 8% and an ownership blocker of 4.99%. In addition, Mr. Struve received a five-year warrant to acquire 1,785,714 shares of common stock at $0.70 per share. On August 14, 2017, the price of the Series C Stock were adjusted to $0.25 per share pursuant to the documents governing such instruments. On September 30, 2021 and 2020 there are 1,785,715 Series C Preferred shares outstanding. On January 5, 2021, the Company extended the warrant expiration date to August 4, 2023.
As of September 30, 2021 and 2020, the Company has $750,000 of Series D Preferred Stock outstanding with Clayton A. Struve, an accredited investor. On August 14, 2017, the price of the Series D Stock were adjusted to $0.25 per share pursuant to the documents governing such instruments. The Series D Preferred Stock is convertible into shares of common stock at a price of $0.25 per share or by multiplying the number of Series D Preferred Stock shares by the stated value and dividing by the conversion price then in effect, subject to certain diluted events, and has the right to vote the number of shares of common stock the Series D Preferred Stock would be issuable on conversion, subject to a 4.99% blocker. The Preferred Series D has an annual yield of 8% The Series D Preferred Stock is convertible into shares of common stock at a price of $0.25 per share or by multiplying the number of Series D Preferred Stock shares by the stated value and dividing by the conversion price then in effect, subject to certain diluted events, and has the right to vote the number of shares of common stock the Series D Preferred Stock would be issuable on conversion, subject to a 4.99% blocker. The Preferred Series D has an annual yield of 8% if and when dividends are declared.
Series F Preferred Stock
On August 1, 2018, the Company filed with the State of Nevada a Certificate of Designation establishing the Designations, Preferences, Limitations and Relative Rights of Series F Preferred Stock. The Designation authorized 500 shares of Series F Preferred Stock. The Series F Preferred Stock shall only be issued to the current Board of Directors on the date of the Designation’s filing and is not convertible into common stock. As set forth in the Designation, the Series F Preferred Stock has no rights to dividends or liquidation preference and carries rights to vote 100,000 shares of common stock per share of Series F upon a Trigger Event, as defined in the Designation. A Trigger Event includes certain unsolicited bids, tender offers, proxy contests, and significant share purchases, all as described in the Designation. Unless and until a Trigger Event, the Series F shall have no right to vote. The Series F Preferred Stock shall remainissued and outstanding until the date which is 731 days after the issuance of Series F Preferred Stock (“Explosion Date”), unless a Trigger Event occurs, in which case the Explosion Date shall be extended by 183 days. As of September 30, 2021 and 2020, there are no Series F shares outstanding.
Securities Subject to Price Adjustments
In the future, if the Company sells its common stock at a price below $0.25 per share, the exercise price of 8,108,356 outstanding shares of Series C and D Preferred Stock that adjust below $0.25 per share pursuant to the documents governing such instruments. In addition, the conversion price of Convertible Notes Payable of $16,464,066 or 16,124,764 common shares (9,020,264 common shares at $0.25 per share and 7,104,500 at $2.00) and the exercise price of additional outstanding warrants to purchase 10,384,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments. Warrants totaling 4,487,207 would adjust below $1.20 per share pursuant to the documents governing such instruments. Warrants totaling 3,954,625 would adjust below $2.40 per share pursuant to the documents governing such instruments.
Common Stock
All of the offerings and sales described below were deemed to be exempt under Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act. No advertising or general solicitation was employed in offering the securities, the offerings and sales were made to a limited number of persons, all of whom were accredited investors and transfer was restricted by the company in accordance with the requirements of Regulation D and the Securities Act. All issuances to accredited and non-accredited investors were structured to comply with the requirements of the safe harbor afforded by Rule 506 of Regulation D, including limiting the number of non-accredited investors to no more than 35 investors who have sufficient knowledge and experience in financial and business matters to make them capable of evaluating the merits and risks of an investment in our securities.
Year Ended September 30, 2021
The Company issued 6,091,960 shares of common stock related to the automatic conversion of Convertible Notes and interest from a private placement to accredited investors in 2020. The Convertible Notes and interested were automatically converted to Common Stock at $1.00 per share on the one year anniversary starting on October 17, 2020.
The Company issued 3,676,542 shares of common stock at an average price of 0.582 per share related to the exercise of warrants.
The Company issued 97,000 shares related to services. The shares were valued at the fair market value of $202,820.
The Company issued 16,875 shares related to the exercise of stock option grants at $1.38 per share.
| F-33 |
| Table of Contents |
The Company issued 480,600 shares of common stock at an average price of $2.00 per share or $961,200 related to the conversion of Particle Simple Agreements for Future Equity into the Company’s common shares.
Year Ended September 30, 2020
On November 9, 2019, a former employee exercised stock option grants on a cashless basis. The former employee received 73,191 shares of common stock for vested stock option grants. The stock option grant had an exercise price of $0.25 per share.
During the year ended September 30, 2020, the Company issued 550,000 shares of restricted common stock for services. The shares were issued were valued at $1.90 per share, the market price of our common stock, or $1,045,000.
During the year ended September 30, 2020, the Company issued 4,581,917 shares of common stock related to the automatic conversion of Convertible Notes and interest from a private placement to accredited investors in 2019. The Convertible Notes and interested were automatically converted to Common Stock at $1.00 per share on the one year anniversary starting on February 15, 2020.
During the year ended September 30, 2020, the Company issued 733,588 shares of common stock at $0.889 per share related to the exercise of warrants.
On July 1, 2020, the Company entered into a Settlement Agreement and General Mutual Release with a shareholder of the Company. On July 6, 2020, the shareholder paid $125,000 us and we issued 500,000 shares of common stock. We accrued for the loss on debt settlement of $825,000 as of June 30, 2020 which represents the difference between the fair market value of the stock and $125,000 paid by the shareholder.
Warrants to Purchase Common Stock
Year Ended September 30, 2021
The Company issued warrants to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is immediately vested and exercisable on a cash or cashless basis at $1.53 per share and was valued using a Black-Scholes model at $1,811,691.
During the year ended September 30, 2021, the Company issued warrants to five directors and service providers for 269,510 shares of common stock. The five year warrant is convertible at $1.918 per share and was valued using a Black-Scholes model at $735,745.
The Convertible Notes issued during the year ended September 30, 2021 are initially convertible into 7,104,500 shares of Common Stock, subject to certain adjustments, and the Warrants are initially exercisable for 3,552,250 shares of Common Stock.
The Company issued 3,676,542 shares of common stock at an average price of $0.582 per share related to the exercise of warrants.
Warrants to exercise 384,359 shares of common stock were forfeited at an average of $1.155 per share.
The Company also issued a five year warrant to Boustead Securities LLC for 43,254 shares of the Company’s common stock related to the conversion of Particle Simple Agreements for Future Equity into the Company’s common shares. The warrant is exercisable at $2.40 per share. The warrant was valued at $1.641 per share or $70,980.
Year Ended September 30, 2020
The following warrant transactions occurred during the year ended September 30, 2020:
During the year ended September 30, 2020, the Company issued 733,588 shares of common stock at $0.952 per share and cancelled warrants to purchase 507,560 shares of common stock at $1.120 per share to related to the exercise of warrants.
During the year ended September 30, 2020, the Company issued 75,000 shares of common stock at $1.95 per share. The warrant was valued at $1.770 per share.
Convertible Debt Offering Warrants
The Warrants issued for the 2020 convertible Debt Offering were granted on a 1:0.5 basis (one-half Warrant for each full share of Common Stock into which the Convertible Notes are convertible). The Warrants have a five-year term and an exercise price equal to 120% of the per share conversion price of the Qualified Financing or other mandatory conversion.
Warrants issued in connection with 2020 convertible debt offering are initially exercisable for 2,819,750 shares of Common Stock at an exercise price of $1.20 per share of Common Stock, also subject to certain adjustments.
| F-34 |
| Table of Contents |
In connection with the 2020 convertible debt offering, the placement agent for the Convertible Notes and the Warrants received warrants to 615,675 shares of the Company’s common stock, all based on 8% of gross proceeds to the Company.
A summary of the warrants outstanding as of September 30, 2021 were as follows:
|
|
| September 30, 2021 |
|
|||||
|
|
|
|
|
| Weighted |
|
||
|
|
|
|
|
| Average |
|
||
|
|
|
|
|
| Exercise |
|
||
|
|
| Shares |
|
| Price |
|
||
| Outstanding at beginning of period |
|
| 20,016,367 |
|
| $ | 0.556 |
|
| Issued |
|
| 6,608,789 |
|
|
| 2.117 |
|
| Exercised |
|
| (3,676,542 | ) |
|
| (0.582 | ) |
| Forfeited |
|
| (384,359 | ) |
|
| (1.155 | ) |
| Expired |
|
| - |
|
|
| - |
|
| Outstanding at end of period |
|
| 22,564,255 |
|
| $ | 0.998 |
|
| Exerciseable at end of period |
|
| 22,564,255 |
|
|
|
|
|
The following table summarizes information about warrants outstanding and exercisable as of September 30, 2021:
|
|
|
| September 30, 2021 |
|
||||||||||||||
|
|
|
| Weighted |
|
| Weighted |
|
|
|
| Weighted |
|
||||||
|
|
|
| Average |
|
| Average |
|
|
|
| Average |
|
||||||
| Number of |
|
| Remaining |
|
| Exercise |
|
| Shares |
|
| Exercise |
|
|||||
| Warrants |
|
| Life ( In Years) |
|
| Price |
|
| Exerciseable |
|
| Price |
|
|||||
|
| 10,829,381 |
|
|
| 1.24 |
|
| $ | 0.250 |
|
|
| 10,829,381 |
|
| $ | 0.250 |
|
|
| 847,742 |
|
|
| 0.12 |
|
|
| 1.000 |
|
|
| 847,742 |
|
|
| 1.000 |
|
|
| 6,512,207 |
|
|
| 3.32 |
|
| 1.20-1.85 |
|
|
| 6,512,207 |
|
| 1.20-1.85 |
|
||
|
| 4,364,925 |
|
|
| 4.50 |
|
| 2.00-2.40 |
|
|
| 4,364,925 |
|
| 2.00-2.40 |
|
||
|
| 10,000 |
|
|
| 1.75 |
|
|
| 4.080 |
|
|
| 10,000 |
|
|
| 4.080 |
|
|
| 22,564,255 |
|
|
| 3.49 |
|
| $ | 0.998 |
|
|
| 22,564,255 |
|
| $ | 0.998 |
|
The significant weighted average assumptions relating to the valuation of the Company’s warrants for the year ended September 30, 2021 were as follows:
| Dividend yield |
|
| 0 | % |
| Expected life |
| 3-5 years |
|
|
| Expected volatility |
|
| 140 | % |
| Risk free interest rate |
|
| 0.37 | % |
There were vested and in the money warrants of 22,554,255 with an aggregate intrinsic value of $34,314,540.
10. STOCK INCENTIVE PLANS
Know Labs, Inc. Stock Incentive Plan
On January 23, 2019, the Board approved an amendment to its 2011 Stock Incentive Plan increasing the number of shares of common stock reserved under the Incentive Plan from 2,200,000 to 2,500,000 to common shares. On May 22, 2019, the Compensation Committee approved an amendment to its 2011 Stock Incentive Plan increasing the number of shares of common stock reserved under the Incentive Plan from 2,500,000 to 3,000,000 to common shares. On November 23, 2020, the Board of Directors increased the size of the stock available under the Stock Option Plan by 9,750,000 shares. This increase is based on an industry peer group study.
On October 15, 2021, at the annual shareholder meeting held on October 15, 2021, the 2021 Equity Incentive Plan was adopted and approved, increasing size of the stock available under the Stock Option Plan to 20,000,000 shares. On December 10, 2021, the Company filed a registration statement on Form S-8 that registered 34,650,120 shares issued under the 2011 Stock Incentive Plan and 2021 Equity Incentive Plan.
| F-35 |
| Table of Contents |
Year Ended September 30, 2021
During the year ended September 30, 2021, the Company issued stock option grants to seventeen employees and consultants totaling 10,650,745 shares of common stock at an average price of $1.766 per share. The stock option grants expire in five years. Stock option grants totaling 9,145,745 vest when earned based on certain performance criteria and 1,505,000 option grants vest quarterly over 4 years, with nothing vesting in the first two quarters. No stock compensation expense has been recorded through September 30, 2021 for those options with performance milestones.
During the year ended September 30, 2021, two consultants exercised stock option grants for 20,625 shares at $1.359 per share.
During the year ended September 30, 2021, an employee forfeited a stock option grant for 120,000 shares at $3.30 per share.
Year Ended September 30, 2020
The Company had the following stock option transactions during the year ended September 30, 2020:
During the year ended September 30, 2020, the Company granted stock option grants to executives, directors and consultants for 3,085,000 shares with a weighted average exercise price of $1.142 per share. The grants expire in five years and generally vest quarterly over four years. Stock option grants totaling 2,630,000 shares of common stock are performance stock option grants and are not vested until the performance is achieved. No stock compensation expense has been recorded through September 30, 2020 for those options with performance milestones.
During the year ended September 30, 2020, executives and employees voluntarily cancelled stock option grants for 2,739,477 shares with a weighted average exercise price of $2.593 per share.
On November 9, 2019, a former employee exercised stock option grants on a cashless basis. The former employee received 73,191 shares of common stock for vested stock option grants totaling 93,750 shares. The stock option grant had an exercise price of $0.25 per share.
There are currently 15,315,120 (including unearned stock option grants totaling 11,775,745 shares related to performance milestones) options to purchase common stock at an average exercise price of $1.565 per share outstanding as of September 30, 2021 under the 2011 Stock Incentive Plan. The Company recorded $1,028,522 and $1,702,085 of compensation expense, net of related tax effects, relative to stock options for the year ended September 30, 2021 and 2020 and in accordance with ASC 718. As of September 30, 2021, there is approximately $1,312,936, net of forfeitures, of total unrecognized costs related to employee granted stock options that are not vested. These costs are expected to be recognized over a period of approximately 3.82 years.
Stock option activity for the years ended September 30, 2021 and 2020 was as follows:
|
|
|
|
|
| Weighted Average |
|
|
|
|
|||
|
|
| Options |
|
| Exercise Price |
|
| $ |
|
|||
| Outstanding as of September 30, 2019 |
|
| 4,532,668 |
|
| $ | 2.025 |
|
| $ | 9,180,369 |
|
| Granted |
|
| 3,085,000 |
|
|
| 1.142 |
|
|
| 3,522,400 |
|
| Exercised |
|
| (73,191 | ) |
|
| (0.250 | ) |
|
| (18,298 | ) |
| Forfeitures |
|
| (2,739,477 | ) |
|
| (2.593 | ) |
|
| (7,103,921 | ) |
| Outstanding as of September 30, 2020 |
|
| 4,805,000 |
|
|
| 1.161 |
|
|
| 5,580,550 |
|
| Granted |
|
| 10,650,745 |
|
|
| 1.766 |
|
|
| 18,807,990 |
|
| Exercised |
|
| (20,625 | ) |
|
| (1.359 | ) |
|
| (28,031 | ) |
| Forfeitures |
|
| (120,000 | ) |
|
| (3.300 | ) |
|
| (396,000 | ) |
| Outstanding as of September 30, 2021 |
|
| 15,315,120 |
|
| $ | 1.565 |
|
| $ | 23,964,509 |
|
| F-36 |
| Table of Contents |
The following table summarizes information about stock options outstanding and exercisable as of September 30, 2021:
|
|
|
|
|
|
| Weighted |
|
| Weighted |
|
|
|
|
| Weighted |
|
||||||
|
|
|
|
|
|
| Average |
|
| Average |
|
|
|
|
| Average |
|
||||||
| Range of |
|
| Number |
|
| Remaining Life |
|
| Exercise Price |
|
| Number |
|
| Exercise Price |
|
||||||
| Exercise Prices |
|
| Outstanding |
|
| In Years |
|
| Outstanding |
|
| Exerciseable |
|
| Exerciseable |
|
||||||
| $ | 0.25 |
|
|
| 230,000 |
|
|
| 1.71 |
|
| 0.250 |
|
|
| 172,500 |
|
| $ | 0.250 |
|
|
| 1.10-1.25 |
|
|
| 3,074,375 |
|
|
| 3.15 |
|
|
| 1.108 |
|
|
| 445,456 |
|
|
| 1.105 |
|
|
| 1.28-1.53 |
|
|
| 9,480,745 |
|
|
| 3.58 |
|
|
| 1.499 |
|
|
| 993,750 |
|
|
| 1.307 |
|
|
| 1.79-3.67 |
|
|
| 2,530,000 |
|
|
| 4.64 |
|
|
| 2.192 |
|
|
| 108,750 |
|
|
| 1.895 |
|
|
|
|
|
|
|
| 15,315,120 |
|
|
| 3.82 |
|
| $ | 1.565 |
|
|
| 1,720,456 |
|
| $ | 1.316 |
|
There stock option grants of 15,315,120 shares as of September 30, 2021 with an aggregate intrinsic value of $14,916,905.
Particle, Inc. Stock Incentive Plan
On May 21, 2020, Particle approved a 2020 Stock Incentive Plan and reserved 8,000,000 shares under the Plan. The Plan requires vesting annually over four years, with no vesting in the first two quarters.
During the year ended September 30, 2021, Particle approved a stock option grant to nine employees and consultants totaling 1,900,000 shares at an average of $0.80 per share. The stock option grant vests (i) 33.3% with the first shipment; (ii) 33.3% with $50 million in sales are achieved; and (iii) 33.4% after $200 million in sales are achieved.
During the year ended September 30, 2021, Particle approved stock option grants to employees totaling 550,000 shares at $0.80 per share. The stock option grants vest annually over four years, with no vesting in the first two quarters.
As of September 30, 2021 the 2020 Stock Incentive Plan, was terminated and all stock option grants were cancelled by the participants. The Company recorded $197,553 and $833,771 of compensation expense, net of related tax effects, relative to stock options for the years ended September 30, 2021 and 2020 and in accordance with ASC 718.
11. OTHER SIGNIFICANT TRANSACTIONS WITH RELATED PARTIES
Transactions with Clayton Struve
See Notes 7, 9 and 15 for related party transactions with Clayton A. Struve.
On January 5, 2021, the Company extended the warrant expiration date to August 4, 2023 with Clayton A. Struve, a major investor in the Company:
| Warrant No./Class |
| Issue Date |
| No. Warrant Shares |
|
| Exercise Price |
|
| Original Expiration Date |
| Amended Expiration Date | |||
| Clayton Struve Warrant Series C Warrant W98 |
| 08-04-2016 |
|
| 1,785,715 |
|
| $ | 0.25 |
|
| 08-04-2021 |
| 08-04-2023 | |
| Clayton Struve Warrant Series F Warrant F-1 |
| 11-14-2016 |
|
| 187,500 |
|
| $ | 0.25 |
|
| 11-13-2021 |
| 11-13-2023 | |
| Clayton Struve Warrant Series F Warrant F-2 |
| 12-19-2016 |
|
| 187,500 |
|
| $ | 0.25 |
|
| 12-18-2021 |
| 12-18-2023 | |
On January 28, 2021, Clayton A. Struve exercised warrants on a cashless basis for 889,880 shares of common stock at $0.25 per share, including warrants for 187,500 and 187,500 that were just extended as discussed above.
The Company owes Clayton A. Struve $1,071,000 under convertible promissory or OID notes. On November 8, 2021, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to March 31, 2022.
Mr. Struve invested $1,000,000 in the Debt Offering which closed in May 2019. On March 18, 2020, Mr. Struve received 1,080,000 shares of common stock related to the automatic conversion of the $1,000,000 invested in the Debt Offering.
| F-37 |
| Table of Contents |
Related Party Transactions with Ronald P. Erickson
See Notes 9, 10, 12 and 15 for related party transactions with Ronald P. Erickson.
On October 4, 2019, Ronald P. Erickson voluntarily cancelled a stock option grant for 1,000,000 shares with an exercise price of $3.03 per share. The grant was related to performance and was not vested.
On November 4, 2019, the Company granted a stock option grant to Ronald P. Erickson for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon uplisting to the NASDAQ or NYSE exchanges.
On January 1, 2020, the Company issued 100,000 shares of restricted common stock to Ronald P. Erickson. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $1.90 per share, the market price of our common stock, or $190,000.
On June 1, 2020, Mr. Erickson received a salary of $10,000 per month for work on Particle, Inc. This salary was cancelled as of August 15, 2021.
On July 2, 2020, Particle issued a stock option grant for 1,500,000 shares at $0.10 per share to Ronald P. Erickson. The stock option grant vests (i) 33.3% upon issuance; (ii) 33.3% after the first sale; and (iii) 33.4% after one million in sales are achieved. The stock option grant was forfeited as of September 30, 2021.
On December 15, 2020, the Company issued a fully vested warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is exercisable for cash or non-cash at $1.53 per share and was valued using a Black-Scholes model at $1,811,691.
On December 15, 2020, the Company issued two stock option grants to Ronald P. Erickson, one for 1,865,675 shares and one for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria.
Mr. Erickson and/or entities with which he is affiliated also have accrued compensation, travel and interest of approximately $421,599 and $597,177 as of September 30, 2021 and 2020, respectively.
During the year ended September 30, 2021, the Company paid $272,500 of salaries to Mr. Erickson that were previously accrued and reported but were deferred.
On September 30, 2021, the Company approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to March 31, 2022.
Related Party Transaction with Phillip A. Bosua
See Notes 9, 10 and 12 for related party transactions with Phillip A. Bosua.
On October 4, 2019, Philip A. Bosua voluntarily cancelled a stock option grant for 1,000,000 shares with an exercise price of $3.03 per share. The grants was related to performance and was not vested.
On November 4, 2019, the Company granted a stock option grant to Philip A. Bosua for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon FDA approval of the UBAND blood glucose monitor.
On January 1, 2020, the Company issued 150,000 shares of restricted common stock to Phillip A. Bosua. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $1.90 per share, the market price of the Company’s common stock, or $285,000.
On June 1, 2020, Mr. Bosua received a salary of $10,000 per month for work on Particle, Inc. This salary was cancelled as of August 15, 2021.
On July 2, 2020, Particle issued a stock option grant for 1,500,000 shares at $0.10 per share to Philip A. Bosua. The stock option grant vests (i) 33.3% upon issuance; (ii) 33.3% after the first sale; and (iii) 33.4% after one million in sales are achieved. The stock option grant was forfeited as of September 30, 2021.
| F-38 |
| Table of Contents |
On December 15, 2020, the Company issued two stock option grant to Phillip A. Bosua, one for 2,132,195 shares and one for 2,132,200 shares at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria.
On March 18, 2021, the Company approved a $250,000 bonus for Mr. Bosua. The bonus was paid during April 2021.
Stock Issuances and Cancellations to Named Executive Officers and Directors
On November 4, 2019, the Company granted stock option grants to two directors totaling 105,000 shares with an exercise price of $1.10 per share. The stock option grants expire in five years. The stock option grants vested immediately.
On January 1, 2020, the Company issued 120,000 shares of restricted common stock to three directors. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $1.90 per share, the market price of the Company’s common stock, or $228,000.
On January 15, 2021, the Company issued 30,000 shares each to three directors shares at an exercise price of $2.00 per share.
On January 15, 2021, the Company issued 20,000 warrants to purchase common stock each to three directors shares at $2.00 per share. The warrants expire on January 15, 2026.
12. COMMITMENTS, CONTINGENCIES AND LEGAL PROCEEDINGS
Legal Proceedings
The Company may from time to time become a party to various legal proceedings arising in the ordinary course of our business. The Company is currently not a party to any pending legal proceeding that is not ordinary routine litigation incidental to our business.
Employment Agreement with Phillip A. Bosua, Chief Executive Officer
Phillip A. Bosua was appointed our Chief Executive Officer on April 10, 2018. Previously, Mr. Bosua served as the Company’s Chief Product Officer since August 2017. The Company entered into a Consulting Agreement with Mr. Bosua’s company, Blaze Clinical on July 7, 2017. From September 2012 to February 2015, Mr. Bosua was the founder and Chief Executive Officer of LIFX Inc. (where he developed and marketed an innovative “smart” light bulb) and from August 2015 until February 2016 was Vice President Consumer Products at Soraa (which markets specialty LED light bulbs). From February 2016 to July 2017, Mr. Bosua was the founder and CEO of RAAI, Inc. (where he continued the development of his smart lighting technology). From May 2008 to February 2013 he was the Founder and CEO of LimeMouse Apps, a leading developer of applications for the Apple App Store.
On April 10, 2018, we entered into an Employment Agreement with Mr. Bosua reflecting his appointment as Chief Executive Officer. The Employment Agreement is for an initial term of 12 months (subject to earlier termination) and will be automatically extended for additional 12-month terms unless either party notifies the other party of its intention to terminate the Employment Agreement with at least ninety (90) days prior to the end of the Initial Term or renewal term. Mr. Bosua was paid a base salary of $225,000 per year, received 500,000 shares of common stock valued at $0.33 per share and may be entitled to bonuses and equity awards at the discretion of the Board or a committee of the Board. The Employment Agreement provides for severance pay equal to 12 months of base salary if Mr. Bosua is terminated without “cause” or voluntarily terminates his employment for “good reason.” During the years ended September 30, 2021 and 2020, the Compensation Committee and the Board compensated Phillip A. Bosua, its Chief Executive Officer, with an annual salary of $240,000 from October 1, 2019 to May 1, 2020. May 1, 2020 to March 31, 2021, the annual compensation was $260,000. From April 1, 2021 to September 30, 2021, the annual compensation was $350,000.
The Compensation Committee and the Board of Particle, Inc. compensated Phillip A. Bosua with an annual salary of $120,000 from June 1, 2020 to August 15, 2021.
Mr. Bosua will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements.
If the Company terminates Mr. Bosua’s employment at any time prior to the expiration of the Term without Cause, as defined in the Employment Agreement, or if Mr. Bosua terminates his employment at any time for “Good Reason” or due to a “Disability,” Mr. Bosua will be entitled to receive (i) his Base Salary amount for one year; and (ii) medical benefits for eighteen months.
| F-39 |
| Table of Contents |
Employment Agreement with Ronald P. Erickson, Chairman of the Board and Interim Chief Financial Officer
On April 10, 2018, we entered into an Amended Employment Agreement for Ronald P. Erickson which amends the Employment Agreement dated July 1, 2017. The Agreement expires March 21, 2019. automatically be extended for additional one (1) year periods unless either Party delivers written notice of such Party’s intention to terminate this Agreement at least ninety (90) days prior to the end of the Initial Term or renewal term.
During the years ended September 30, 2021 and 2020, the Compensation Committee and the Board compensated Ronald P. Erickson, its Chairman of the Board and Interim Financial Officer, with an annual salary of $195,000 from October 1, 2019 to May 1, 2020. From May 1, 2020 to March 31, 2021, the annual compensation was $215,000. From April 1, 2021 to September 30, 2021, the annual compensation was $300,000. The Compensation Committee and the Board of Particle Inc. compensated Ronald P. Erickson with an annual salary of $120,000 from June 1, 2020 to August 15, 2021.
Mr. Erickson will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements.
If the Company terminates Mr. Erickson’s employment at any time prior to the expiration of the Term without Cause, as defined in the Employment Agreement, or if Mr. Erickson terminates his employment at any time for “Good Reason” or due to a “Disability,” Mr. Erickson will be entitled to receive (i) his Base Salary amount for one year; and (ii) medical benefits for eighteen months.
Properties and Operating Leases
The Company is obligated under the following leases for its various facilities.
Corporate Offices
On April 13, 2017, the Company leased its executive office located at 500 Union Street, Suite 810, Seattle, Washington, USA, 98101. The Company leases 943 square feet and the current net monthly payment is $3,334. The monthly payment increases approximately 3% each year and the lease expires on May 31, 2022.
Lab Facilities and Executive Offices
On February 1, 2019, the Company leased its lab facilities and executive offices located at 915 E Pine Street, Suite 212, Seattle, WA 98122. We lease 2,642 square feet and the net monthly payment at September 30, 2021 is $8,697. The monthly payment increases approximately 3% annually each year on July 1. The lease expires on June 30, 2024.
13. INCOME TAXES
The Company has incurred losses since inception, which have generated net operating loss carryforwards. The net operating loss carryforwards arise from United States sources.
Losses arising from United States taxable operations were approximately $6.5 million and $5.1 million for the years ended September 30, 2021 and 2020.
The Company has Federal net operating loss carryforwards of approximately $43.8 million which expire in 2028-2041. Because it is not more likely than not that sufficient tax earnings will be generated to utilize the net operating loss carryforwards, a corresponding valuation allowance equal to 100% of the gross deferred tax asset of approximately $9.7 million and $8.0 million was established as of September 30, 2021 and 2020. The Company does not recognize the majority of state tax loss operating loss carryforwards as a deferred tax asset given it no longer has any operation in that state.
Under the Tax Reform Act of 1986, the amounts of, and benefits from, net operating losses may be limited in certain circumstances, including a change in control. Section 382 of the Internal Revenue Code generally imposes an annual limitation on the amount of net operating loss carryforwards that may be used to offset taxable income when a corporation has undergone significant changes in its stock ownership. There can be no assurance that the Company will be able to utilize any net operating loss carryforwards in the future. The Company is subject to possible tax examination for the years 2016 through 2021.
| F-40 |
| Table of Contents |
The principal components of the Company’s deferred tax assets at September 30, 2021 and 2020 are as follows:
|
|
| 2021 |
|
| 2020 |
|
||
| Net operating loss carryforward |
| $ | 8,051,000 |
|
| $ | 6,536,000 |
|
| Stock based compensation |
|
| 975,000 |
|
|
| 1,196,000 |
|
| Intangibles |
|
| 276,000 |
|
|
| 305,000 |
|
| Accruals and reserves |
|
| 399,000 |
|
|
| 11,000 |
|
| Total deferred tax asset |
|
| 9,701,000 |
|
|
| 8,048,000 |
|
| Valuation allowance |
|
| (9,701,000 | ) |
|
| (8,048,000 | ) |
| Net deferred tax assets |
| $ | - |
|
| $ | - |
|
| Change in valuation allowance during the year |
| $ | (1,653,000 | ) |
| $ | (1,092,357 | ) |
A reconciliation of the United States Federal Statutory rate to the Company’s effective tax rate for the years ended September 30, 2021 and 2020 are as follows. For the year ended September 30, 2021 and 2020, the Company’s effective tax rate differs from the federal statutory rate principally due to non deductible warrant interest expense plus an increase in the deferred tax asset valuation allowance.
|
|
| 2021 |
|
| 2020 |
|
||
| Income tax provision at statutory rate |
|
| -21.0 | % |
|
| -21.0 | % |
| Warrant interest expense |
|
| 12 | % |
|
| 9 | % |
| Change in valuation allowance |
|
| 7 | % |
|
| 9 | % |
| Prior year true up |
|
| 2 | % |
|
| 3 | % |
| Effective tax rate |
|
| 0 | % |
|
| 0 | % |
As of September 30, 2021, there were no uncertain tax positions. Management does not anticipate any future adjustments in the next twelve months which would result in a material change to its tax position. For the years ended September 30, 2021 and 2020, the Company did not have any interest and penalties.
14. SEGMENT REPORTING
The management of the Company considers the business to currently have two operating segments (i) the development of the Bio-RFID™” and “ChromaID™” technologies; (ii) Particle, Inc. technology. ) TransTech, a distributor of products for employee and personnel identification and authentication was shut down on June 30, 2020. Particle commenced operations in the three months ended June 30, 2020.
The reporting for the year ended September 30, 2021 and 2020 was as follows (in thousands):
|
|
|
|
| Gross |
|
| Operating |
|
| Segment |
|
|||||
| Segment |
| Revenue |
|
| Margin |
|
| (Loss) |
|
| Assets |
|
||||
| Year Ended September 30, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
| Development of the Bio-RFID™” and “ChromaID™” technologies |
| $ | - |
|
| $ | - |
|
| $ | (9,373 | ) |
| $ | 12,867 |
|
| Particle, Inc. technology |
|
| - |
|
|
| - |
|
|
| (1,073 | ) |
|
| 22 |
|
| TransTech distribution business |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
| Total segments |
| $ | - |
|
| $ | - |
|
| $ | (10,446 | ) |
| $ | 12,889 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended September 30, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Development of the Bio-RFID™” and “ChromaID™” technologies |
| $ | - |
|
| $ | - |
|
| $ | (5,481 | ) |
| $ | 4,360 |
|
| Particle, Inc. technology |
|
| - |
|
|
| - |
|
|
| (1,280 | ) |
|
| 322 |
|
| TransTech distribution business |
|
| 122 |
|
|
| 70 |
|
|
| (65 | ) |
|
| - |
|
| Total segments |
| $ | 122 |
|
| $ | 70 |
|
| $ | (6,826 | ) |
| $ | 4,682 |
|
During years ended September 30, 2021 and 2020, the Company incurred non-cash expenses related to operations of $3,979,584 and $2,990,072.
15. SUBSEQUENT EVENTS
The Company evaluated subsequent events, for the purpose of adjustment or disclosure, up through the date the financial statements were issued. Subsequent to September 30, 2021, there were the following material transactions that require disclosure:
Annual Shareholder Meeting
On October 15, 2021, the Company held its annual shareholder meeting. The Company’s shareholders approved and adopted various motions as detailed in the Company’s Form 8-K that was filed with the SEC on October 19, 2021.
2021 Equity Incentive Plan
On October 15, 2021, at the annual shareholder meeting held on October 15, 2021, the 2021 Equity Incentive Plan was adopted and approved, increasing size of the stock available under the Stock Option Plan to 20,000,000 shares. On December 10, 2021, the Company filed a registration statement on Form S-8 that registered 34,650,120 shares issued under the 2011 Stock Incentive Plan and 2021 Equity Incentive Plan.
| F-41 |
| Table of Contents |
Second Amended and Restated Bylaws
On October 15, 2021, the shareholders of the Company approving the Second Amended and Restated Bylaws effective October 15, 2021.
Lease Modifications
On April 13, 2017, the Company leased its executive office located at 500 Union Street, Suite 810, Seattle, Washington, USA, 98101. The Company leases 943 square feet and the current net monthly payment is $3,334. The monthly payment increases approximately 3% each year and the lease expires on May 31, 2022. On October 31, 2021, the Company extended the lease from June 1, 2022 to May 31, 2023 at $2,986 per month.
On February 1, 2019, the Company leased its lab facilities and executive offices located at 915 E Pine Street, Suite 212, Seattle, WA 98122. The Company leases 2,642 square feet and the net monthly payment at September 30, 2021 is $8,697. The monthly payment increases approximately 3% annually each year on July 1. The lease expires on June 30, 2024. On October 11, 2021, the Company entered into First Amendment of Lease and added 1,030 square feet for year for $1,000 for $5,000 per month. The space will be utilized for clinical trials.
Extension of Convertible Promissory Notes with Clayton A. Struve
On November 8, 2021, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to March 31, 2022.
Certificate of Amendment to Articles of Incorporation
On December 6, 2021, the Company received approval from the State of Nevada for a Certificate of Amendment to the Articles of Incorporation related to the increase in the number of authorized common shares.
AI Revenue
On December 16, 2021, the Company, announced the Company’s Artificial Intelligence (AI) Deep Learning Platform has generated initial revenue of approximately $4.2 million from Non-Fungible Token (NFT) sales.
Note Payable-PPP Loans
On April 30, 2020, the Company received $226,170 under the Paycheck Protection Program of the U.S. Small Business Administration’s 7(a) Loan Program pursuant to the Coronavirus, Aid, Relief and Economic Security Act (CARES Act), Pub. Law 116-136, 134 Stat. 281 (2020). The Company filed the application for the loan forgiveness during the three months ended December 31, 2021 and the Company is expecting approval by the SBA.
On February 1, 2021, the Company received $205,633 under the Paycheck Protection Program of the U.S. Small Business Administration’s 7(a) Loan Program pursuant to the Coronavirus, Aid, Relief and Economic Security Act (CARES Act), Pub. Law 116-136, 134 Stat. 281 (2020). The Company filed the application for the loan forgiveness during the three months ended December 31, 2021 and the Company is expecting approval by the SBA.
Stock Option Exercises and Issuances
The Company issued 803,361 shares of common stock related to warrant and stock option exercises and received $768,830.
The Compensation committee issued stock option grants to seven employees and three consultants for 910,000 shares at an exercise price of $2.09 per share. The stock option grants expire in five years. The stock option grant vests quarterly over four years.
On December 16, 2021, the Company issued a stock option grant to Ronald P. Erickson for 1,000.000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On December 16, 2021, the Company issued a stock option grant to Phillip A. Bosua for 1,300,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On December 16, 2021, the Company approved 30,000 shares each to three directors shares at terms to be determined during January 2022.
On December 16, 2021, the Company issued 20,000 warrants to purchase common stock each to three directors shares at terms to be determined during January 2022.
| F-42 |
| Table of Contents |
3,600,000 Shares
Common Stock

Know Labs, Inc.
______________________
PROSPECTUS
______________________
Underwriter
BOUSTEAD SECURITIES, LLC
September 15, 2022
Until October 10, 2022 (25 days after the date of this prospectus), all dealers that buy, sell or trade our securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.